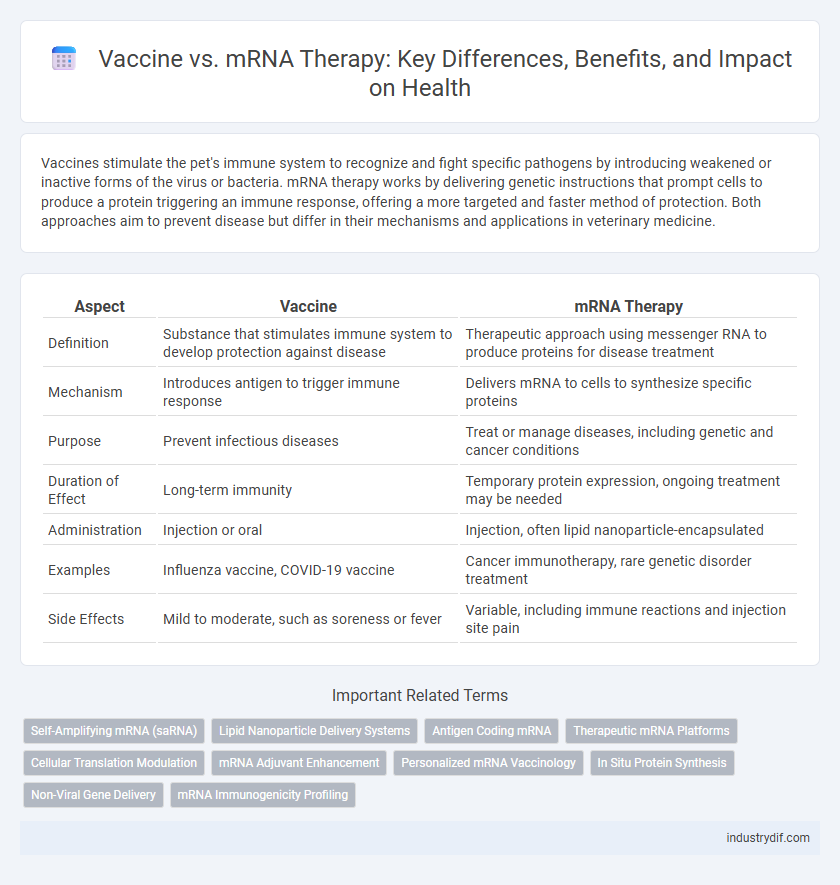
Vaccines stimulate the pet's immune system to recognize and fight specific pathogens by introducing weakened or inactive forms of the virus or bacteria. mRNA therapy works by delivering genetic instructions that prompt cells to produce a protein triggering an immune response, offering a more targeted and faster method of protection. Both approaches aim to prevent disease but differ in their mechanisms and applications in veterinary medicine.
Table of Comparison
| Aspect | Vaccine | mRNA Therapy |
|---|---|---|
| Definition | Substance that stimulates immune system to develop protection against disease | Therapeutic approach using messenger RNA to produce proteins for disease treatment |
| Mechanism | Introduces antigen to trigger immune response | Delivers mRNA to cells to synthesize specific proteins |
| Purpose | Prevent infectious diseases | Treat or manage diseases, including genetic and cancer conditions |
| Duration of Effect | Long-term immunity | Temporary protein expression, ongoing treatment may be needed |
| Administration | Injection or oral | Injection, often lipid nanoparticle-encapsulated |
| Examples | Influenza vaccine, COVID-19 vaccine | Cancer immunotherapy, rare genetic disorder treatment |
| Side Effects | Mild to moderate, such as soreness or fever | Variable, including immune reactions and injection site pain |
Understanding Traditional Vaccines
Traditional vaccines introduce weakened or inactivated pathogens to stimulate the immune system, enabling the body to recognize and combat specific viruses or bacteria. They rely on antigen presentation to trigger antibody production and long-term immunity. This method contrasts with mRNA therapy, which uses genetic instructions to prompt cells to produce target proteins internally, initiating an immune response without exposure to the actual pathogen.
Key Principles of mRNA Therapy
mRNA therapy utilizes synthetic messenger RNA to instruct cells to produce specific proteins that stimulate an immune response or treat diseases, offering targeted and adaptable treatment options. Unlike traditional vaccines that introduce weakened pathogens or protein subunits, mRNA therapies leverage the body's cellular machinery for precise protein synthesis and faster development cycles. Key principles include the use of lipid nanoparticles for efficient mRNA delivery, transient protein expression without altering DNA, and the ability to rapidly tailor therapies for diverse medical applications.
Mechanisms of Action: Vaccines vs mRNA Therapy
Vaccines stimulate the immune system by introducing antigens or weakened pathogens to trigger an adaptive immune response, promoting long-term immunity. mRNA therapy delivers synthetic messenger RNA into cells to produce specific proteins that either replace defective ones or instruct immune responses without using live pathogens. The distinction lies in vaccines priming immune memory via antigen presentation, whereas mRNA therapy directly codes for therapeutic proteins within host cells.
Efficacy and Immune Response Comparisons
mRNA vaccines deliver genetic instructions to cells, prompting them to produce antigens that elicit a strong and targeted immune response, with efficacy rates often exceeding 90% in preventing specific infections like COVID-19. Traditional vaccines use inactivated pathogens or protein subunits to stimulate immunity, which may result in a broader but sometimes less potent immune activation. Clinical studies highlight that mRNA therapies tend to induce higher neutralizing antibody titers and robust T-cell responses compared to conventional vaccine platforms.
Safety Profiles and Side Effects
Vaccine safety profiles are well-established through extensive clinical trials and post-market surveillance, with common side effects including mild injection site pain, fatigue, and low-grade fever. mRNA therapies, while utilizing similar technology, often exhibit a targeted mechanism with fewer systemic reactions but may still cause localized inflammation or temporary flu-like symptoms. Monitoring adverse effects remains crucial for both, ensuring ongoing evaluation of their risk-benefit balance in diverse populations.
Development and Manufacturing Differences
Vaccine development typically involves using inactivated pathogens or protein subunits to stimulate an immune response, whereas mRNA therapy leverages synthetic messenger RNA to instruct cells to produce specific proteins. Manufacturing vaccines often requires cultivating live viruses or proteins in bioreactors, a time-consuming and complex process, while mRNA therapies are produced through rapid cell-free enzymatic synthesis, allowing for faster scalability. The distinct production methods impact storage, distribution, and adaptability to emerging variants, with mRNA platforms offering greater flexibility in design modifications compared to traditional vaccine manufacturing.
Storage and Distribution Challenges
Vaccine distribution faces significant challenges due to stringent cold chain requirements, with mRNA vaccines like Pfizer-BioNTech demanding ultra-low temperatures of around -70degC, complicating storage and transport logistics. In contrast, traditional vaccines often require standard refrigeration between 2degC and 8degC, making them easier to handle but sometimes less effective against rapidly mutating viruses. Advances in mRNA therapy focus on enhancing lipid nanoparticle stability and exploring lyophilization techniques to improve thermal resilience and facilitate broader global distribution.
Regulatory Approvals and Guidelines
Vaccine regulatory approvals prioritize demonstrated safety and efficacy through large-scale clinical trials, with agencies like the FDA and EMA requiring extensive data before authorization. mRNA therapies, while leveraging similar platform technology, undergo distinct regulatory pathways that emphasize product-specific manufacturing processes, targeted disease applications, and long-term safety monitoring. Guidelines for vaccine development often follow established frameworks for infectious diseases, whereas mRNA therapies, particularly in oncology or rare diseases, necessitate adaptive regulatory strategies reflecting innovative treatment modalities.
Current Applications in Infectious Diseases
Vaccines have been pivotal in preventing infectious diseases such as influenza, measles, and COVID-19 by stimulating the immune system to recognize and neutralize pathogens. mRNA therapy, a cutting-edge approach exemplified by COVID-19 vaccines from Pfizer-BioNTech and Moderna, uses messenger RNA to instruct cells to produce specific antigens, triggering an immune response. Current applications of mRNA technology extend beyond vaccines, including promising developments in treatments for viral infections like Zika and cytomegalovirus, showcasing its versatility in infectious disease management.
Future Trends in Vaccine and mRNA Technologies
Future trends in vaccine and mRNA technologies emphasize personalized medicine, with advancements enabling tailored immune responses to individual genetic profiles. Innovative delivery systems such as lipid nanoparticles and self-amplifying mRNA platforms enhance vaccine efficacy and stability, reducing cold chain dependency. Integration of artificial intelligence in vaccine design accelerates the development of next-generation vaccines targeting emerging infectious diseases and chronic conditions.
Related Important Terms
Self-Amplifying mRNA (saRNA)
Self-amplifying mRNA (saRNA) enhances vaccine efficiency by replicating within host cells, requiring lower doses compared to traditional mRNA therapies, which improves immune response and reduces production costs. This innovative approach offers potential for rapid vaccine development against infectious diseases and cancer by amplifying antigen expression without integrating into the host genome.
Lipid Nanoparticle Delivery Systems
Lipid nanoparticle delivery systems enhance the stability and cellular uptake of both vaccines and mRNA therapies by encapsulating nucleic acids, ensuring efficient transport across biological membranes. Their biocompatibility and ability to protect mRNA from enzymatic degradation significantly increase the therapeutic efficacy of lipid nanoparticle-formulated vaccines and personalized mRNA treatments.
Antigen Coding mRNA
Antigen coding mRNA in vaccines instructs cells to produce specific viral proteins, triggering an immune response without using live virus, while mRNA therapy broadly utilizes this mechanism to encode therapeutic proteins for various diseases. The precision of antigen coding mRNA enables targeted immunity and rapid vaccine development against pathogens like SARS-CoV-2.
Therapeutic mRNA Platforms
Therapeutic mRNA platforms utilize synthetic mRNA to instruct cells to produce specific proteins that stimulate immune responses against diseases, including cancer and infectious pathogens. Unlike traditional vaccines, mRNA therapies offer rapid development, customizable treatments, and potential applications beyond prophylaxis, extending into personalized medicine and gene editing.
Cellular Translation Modulation
mRNA vaccines utilize cellular translation mechanisms to produce antigen proteins, effectively training the immune system without altering the host DNA. In contrast, mRNA therapies modulate cellular translation more broadly, aiming to correct or adjust protein synthesis in targeted diseases beyond immune response activation.
mRNA Adjuvant Enhancement
mRNA adjuvant enhancement significantly boosts immune response by improving antigen presentation and activating innate immune pathways, making mRNA-based therapies more effective than traditional vaccines. This approach leverages synthetic mRNA sequences to amplify cytokine production and dendritic cell activation, optimizing immunogenicity without live pathogens.
Personalized mRNA Vaccinology
Personalized mRNA vaccinology tailors vaccine design to individual genetic profiles, enhancing immune response specificity compared to traditional vaccines. This approach uses mRNA therapy to encode patient-specific antigens, advancing precision medicine in infectious disease prevention and cancer immunotherapy.
In Situ Protein Synthesis
mRNA therapy leverages in situ protein synthesis by delivering genetic instructions directly into cells, enabling them to produce therapeutic proteins internally, unlike traditional vaccines that introduce antigens externally to trigger an immune response. This approach enhances precision in targeting diseases by facilitating rapid and controlled protein production within the patient's body.
Non-Viral Gene Delivery
Non-viral gene delivery systems in vaccine and mRNA therapy utilize lipid nanoparticles or polymer-based carriers to transport genetic material into cells, enhancing safety and reducing immunogenicity compared to viral vectors. This approach enables efficient delivery of mRNA vaccines, promoting robust immune responses while minimizing risks associated with viral DNA integration or replication.
mRNA Immunogenicity Profiling
mRNA immunogenicity profiling evaluates the immune response triggered by both vaccines and mRNA therapies, identifying key cytokine production and T-cell activation markers to optimize efficacy and safety. Precise assessment of mRNA-induced innate and adaptive immunity enables tailored therapeutic design, minimizing adverse reactions while enhancing protective outcomes against targeted diseases.
Vaccine vs mRNA Therapy Infographic
 industrydif.com
industrydif.com