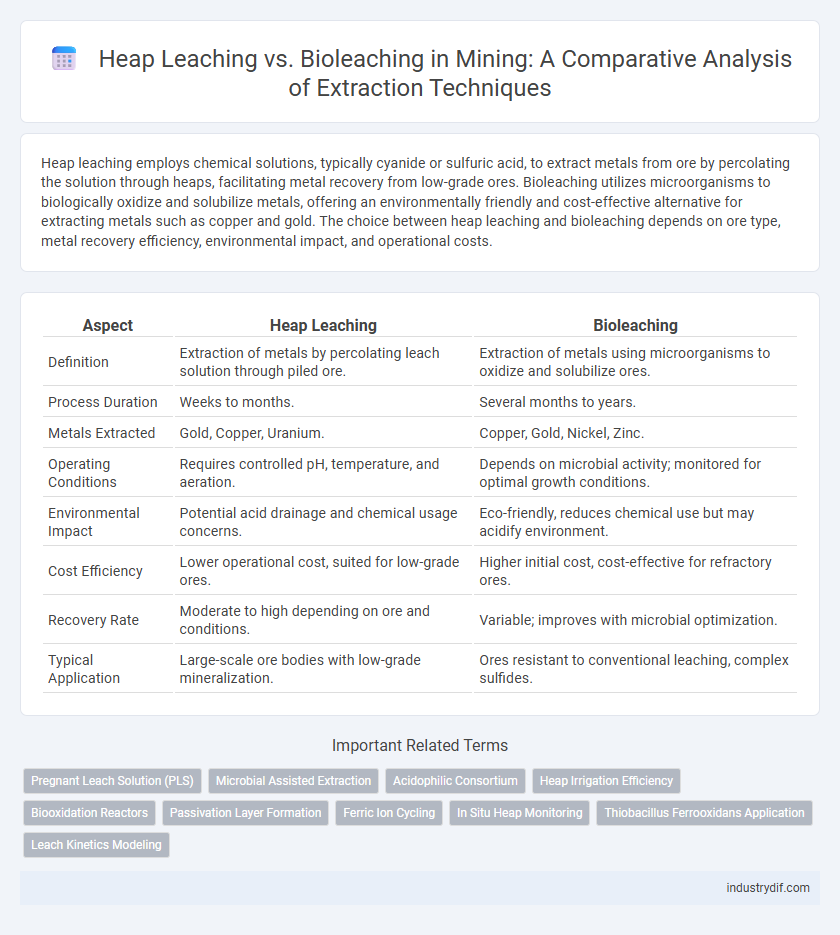
Heap leaching employs chemical solutions, typically cyanide or sulfuric acid, to extract metals from ore by percolating the solution through heaps, facilitating metal recovery from low-grade ores. Bioleaching utilizes microorganisms to biologically oxidize and solubilize metals, offering an environmentally friendly and cost-effective alternative for extracting metals such as copper and gold. The choice between heap leaching and bioleaching depends on ore type, metal recovery efficiency, environmental impact, and operational costs.
Table of Comparison
| Aspect | Heap Leaching | Bioleaching |
|---|---|---|
| Definition | Extraction of metals by percolating leach solution through piled ore. | Extraction of metals using microorganisms to oxidize and solubilize ores. |
| Process Duration | Weeks to months. | Several months to years. |
| Metals Extracted | Gold, Copper, Uranium. | Copper, Gold, Nickel, Zinc. |
| Operating Conditions | Requires controlled pH, temperature, and aeration. | Depends on microbial activity; monitored for optimal growth conditions. |
| Environmental Impact | Potential acid drainage and chemical usage concerns. | Eco-friendly, reduces chemical use but may acidify environment. |
| Cost Efficiency | Lower operational cost, suited for low-grade ores. | Higher initial cost, cost-effective for refractory ores. |
| Recovery Rate | Moderate to high depending on ore and conditions. | Variable; improves with microbial optimization. |
| Typical Application | Large-scale ore bodies with low-grade mineralization. | Ores resistant to conventional leaching, complex sulfides. |
Introduction to Heap Leaching and Bioleaching
Heap leaching is an efficient extraction method that involves stacking ore into heaps and irrigating them with leaching solutions to dissolve valuable metals such as gold, copper, and uranium. Bioleaching utilizes specific microorganisms, including Acidithiobacillus ferrooxidans, to biologically oxidize sulfide minerals and enhance metal recovery in environments where traditional chemical leaching is less effective. Both techniques offer distinct advantages in hydrometallurgical processes by improving metal recovery rates and reducing environmental impact compared to conventional smelting.
Fundamental Principles of Heap Leaching
Heap leaching involves stacking crushed ore into large heaps and irrigating the pile with a leaching solution, typically a cyanide or sulfuric acid solution, to dissolve valuable metals such as gold, copper, or uranium. The fundamental principle relies on the percolation of the leaching solution through the heap, facilitating chemical reactions that extract the target metals from the ore minerals. Efficient heap permeability and optimal solution chemistry are critical factors influencing the metal recovery rate in heap leaching operations.
Core Concepts of Bioleaching
Bioleaching utilizes naturally occurring microorganisms to break down metal sulfides, converting insoluble minerals into soluble metal ions for efficient extraction. This biological process is environmentally friendly and cost-effective, especially for low-grade ores, compared to traditional heap leaching that relies on chemical lixiviants. Key microbes in bioleaching include Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans, which facilitate oxidation and metal solubilization through biochemical mechanisms.
Ore Types Suitable for Each Method
Heap leaching is most effective for low-grade, oxide, and secondary sulfide ores, allowing for economical extraction of metals like gold and copper with minimal processing. Bioleaching excels in treating refractory sulfide ores, particularly chalcopyrite and other primary sulfides, leveraging microbial activity to break down complex minerals. Both techniques optimize metal recovery based on ore mineralogy and economic feasibility.
Chemical and Biological Reactions Involved
Heap leaching involves the application of chemical solutions, typically cyanide or sulfuric acid, to dissolve valuable metals like gold or copper from ore piles, facilitating metal recovery through chemical oxidation and reduction reactions. In contrast, bioleaching employs specific microorganisms, such as Acidithiobacillus ferrooxidans, which biologically oxidize sulfide minerals to release metals via enzymatic activity and metabolic processes. Both methods depend on the breakdown of mineral matrices but differ fundamentally in their reliance on abiotic chemical reactions versus biotic microbial processes for metal solubilization.
Environmental Impact Comparison
Heap leaching involves chemical reagents like cyanide or sulfuric acid, posing risks of soil and water contamination, whereas bioleaching utilizes microorganisms to extract metals, significantly reducing toxic chemical use and environmental hazards. Heap leaching can lead to acid mine drainage and long-term site remediation challenges, while bioleaching promotes biodegradable waste management and lower ecological disturbance. Both methods impact ecosystems, but bioleaching offers a more sustainable, less contaminating alternative in metal recovery processes.
Operational Costs and Efficiency
Heap leaching typically incurs lower operational costs due to simpler infrastructure and reduced energy consumption compared to bioleaching, which requires controlled microbial activity and longer processing times. Bioleaching demonstrates higher recovery efficiency for metals like copper and gold in low-grade ores, benefiting from biological oxidation that enhances metal solubilization. Optimal selection depends on ore type and project scale, balancing heap leaching's cost-effectiveness against bioleaching's superior metal recovery rates.
Metal Recovery Rates and Yields
Heap leaching typically achieves metal recovery rates ranging from 60% to 85%, depending on ore type and processing conditions, with yields influenced by heap permeability and leach solution chemistry. Bioleaching enhances metal recovery rates, often reaching 80% to over 90%, by utilizing microorganisms to oxidize sulfide minerals, which increases metal solubilization efficiency. Comparative studies indicate bioleaching yields higher extraction rates for copper and gold from low-grade ores, making it a preferred method in sustainable mining operations focused on maximizing metal recovery.
Technological Advancements in Leaching Techniques
Heap leaching has advanced with improved heap design and enhanced irrigation systems that optimize reagent distribution and increase metal recovery rates, while bioleaching leverages genetically engineered microorganisms and bioreactor innovations to accelerate metal extraction from low-grade ores. Integration of automation and real-time monitoring technologies in both methods enhances process control, reducing environmental impact and operational costs. These advancements contribute to more sustainable and economically viable mineral processing in the mining industry.
Future Trends and Industry Adoption
Heap leaching is expected to maintain its dominance in large-scale copper and gold extraction due to its cost-effectiveness and scalability, while bioleaching is gaining traction for its environmental benefits and applicability in processing low-grade ores. Future trends indicate a growing integration of biotechnology with heap leaching processes to enhance metal recovery rates and reduce chemical usage. Industry adoption of bioleaching is accelerating, driven by stricter environmental regulations and rising demand for sustainable mining practices.
Related Important Terms
Pregnant Leach Solution (PLS)
Pregnant Leach Solution (PLS) in heap leaching typically contains higher concentrations of target metals due to the direct acidic dissolution of ore, enabling efficient extraction in copper mining. In bioleaching, PLS composition varies with microbial activity, often resulting in slower metal recovery but enhanced environmental sustainability and reduced chemical usage.
Microbial Assisted Extraction
Heap leaching utilizes chemical solutions to extract metals from ore piles, whereas bioleaching leverages microbial-assisted extraction, employing bacteria such as Acidithiobacillus ferrooxidans to biologically oxidize sulfide minerals and enhance metal recovery. Microbial-assisted extraction in bioleaching offers environmentally friendly and cost-effective advantages by improving metal solubilization and reducing the need for harsh chemicals commonly used in heap leaching.
Acidophilic Consortium
Heap leaching utilizes acidophilic consortium bacteria to enhance copper recovery by oxidizing sulfide minerals, while bioleaching relies on these microorganisms to biologically solubilize metals from low-grade ore in a controlled environment. The acidophilic consortium, including Acidithiobacillus ferrooxidans and Leptospirillum spp., plays a crucial role in generating ferric ions and maintaining acidic conditions essential for efficient metal extraction.
Heap Irrigation Efficiency
Heap irrigation efficiency in heap leaching relies on the even distribution of leaching solution to maximize metal recovery from ore piles, whereas bioleaching enhances heap irrigation by utilizing microorganisms to increase permeability and nutrient cycling. Improved heap irrigation in bioleaching promotes faster reaction rates and higher extraction yields, significantly surpassing conventional heap leaching methods.
Biooxidation Reactors
Biooxidation reactors enhance the recovery of precious metals by biologically oxidizing sulfide minerals, facilitating effective gold extraction through bioleaching processes. These reactors maintain controlled temperature, pH, and oxygen levels to optimize microbial activity, improving metal solubilization compared to traditional heap leaching methods.
Passivation Layer Formation
Heap leaching often faces challenges due to the formation of passivation layers on ore surfaces, which inhibit the leaching agents' ability to dissolve valuable metals efficiently. In contrast, bioleaching utilizes bacteria that produce organic acids and enzymes capable of breaking down these passivation layers, enhancing metal recovery rates and reducing environmental impact.
Ferric Ion Cycling
Heap leaching and bioleaching are both hydrometallurgical processes used for extracting metals, but bioleaching involves microorganisms that enhance ferric ion cycling by regenerating Fe3+ from Fe2+, which accelerates metal dissolution. Ferric ion cycling is critical in bioleaching as bacteria such as Acidithiobacillus ferrooxidans oxidize ferrous ions, maintaining a high concentration of ferric ions that act as oxidizing agents to solubilize ores more efficiently than heap leaching alone.
In Situ Heap Monitoring
In situ heap monitoring in heap leaching employs sensors to track variables like temperature, pH, and moisture, enhancing metal recovery efficiency by optimizing lixiviant distribution and heap conditions. Bioleaching incorporates microbial activity monitoring, using biosensors to assess microbial health and metabolic rates, enabling real-time adjustments for improved biooxidation and metal extraction from sulfide ores.
Thiobacillus Ferrooxidans Application
Heap leaching and bioleaching both utilize Thiobacillus ferrooxidans to enhance the extraction of metals from low-grade ores, with heap leaching applying the bacteria in large piles of crushed ore and bioleaching leveraging microbial activity in more controlled environments like bioreactors. Thiobacillus ferrooxidans oxidizes ferrous iron and sulfide minerals, facilitating the solubilization of valuable metals such as copper and gold, making it a critical agent in biohydrometallurgical processes.
Leach Kinetics Modeling
Heap leaching kinetics typically follow a shrinking core model where the rate-controlling step involves the diffusion of the lixiviant through the porous ore matrix, whereas bioleaching kinetics are governed by microbial growth rates and metabolic activity, often modeled using Monod-type equations. Accurate leach kinetics modeling incorporates factors such as ore particle size, temperature, pH, and microbial consortia dynamics to optimize metal recovery rates in both heap and bioleaching processes.
Heap Leaching vs Bioleaching Infographic
 industrydif.com
industrydif.com