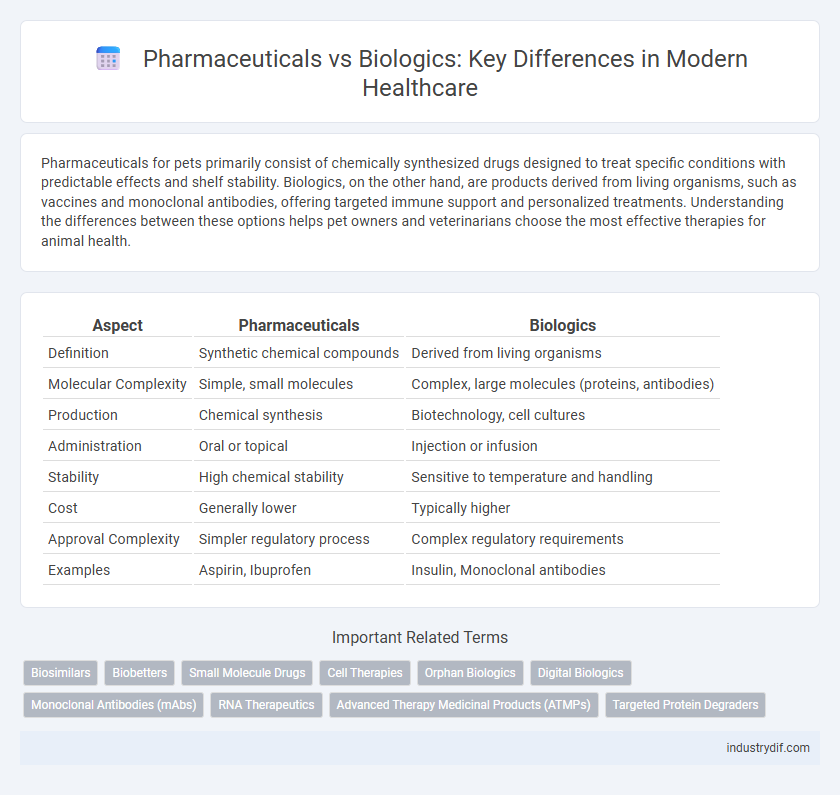
Pharmaceuticals for pets primarily consist of chemically synthesized drugs designed to treat specific conditions with predictable effects and shelf stability. Biologics, on the other hand, are products derived from living organisms, such as vaccines and monoclonal antibodies, offering targeted immune support and personalized treatments. Understanding the differences between these options helps pet owners and veterinarians choose the most effective therapies for animal health.
Table of Comparison
| Aspect | Pharmaceuticals | Biologics |
|---|---|---|
| Definition | Synthetic chemical compounds | Derived from living organisms |
| Molecular Complexity | Simple, small molecules | Complex, large molecules (proteins, antibodies) |
| Production | Chemical synthesis | Biotechnology, cell cultures |
| Administration | Oral or topical | Injection or infusion |
| Stability | High chemical stability | Sensitive to temperature and handling |
| Cost | Generally lower | Typically higher |
| Approval Complexity | Simpler regulatory process | Complex regulatory requirements |
| Examples | Aspirin, Ibuprofen | Insulin, Monoclonal antibodies |
Overview of Pharmaceuticals and Biologics
Pharmaceuticals are chemically synthesized drugs designed to target specific biological pathways and treat various health conditions with precise molecular structures. Biologics, derived from living organisms, include complex molecules like proteins, antibodies, and vaccines, offering targeted therapies for diseases such as cancer and autoimmune disorders. Both categories play critical roles in modern medicine, with pharmaceuticals providing standardized treatments and biologics enabling personalized medical approaches.
Key Differences Between Pharmaceuticals and Biologics
Pharmaceuticals are chemically synthesized drugs with well-defined structures, while biologics are complex molecules derived from living organisms, including proteins and antibodies. Pharmaceuticals typically have simpler manufacturing processes, whereas biologics require advanced biotechnological methods and stringent storage conditions. The regulatory approval for biologics often involves more rigorous clinical trials due to their complexity and variability compared to traditional pharmaceuticals.
Drug Development Processes: Pharmaceuticals vs Biologics
Pharmaceutical drug development involves chemical synthesis to create small molecules with precise structures, enabling controlled dosing and stability, whereas biologics are developed using living cells and complex biological processes to produce large, intricate proteins or antibodies. The manufacturing of biologics requires stringent conditions and bioreactors to maintain cell viability and product consistency, contrasting with the more predictable chemical synthesis and purification methods used for pharmaceuticals. Regulatory pathways differ as well, with biologics often undergoing more extensive characterization and batch-to-batch variability considerations compared to chemically synthesized drugs.
Manufacturing Techniques in Pharmaceuticals and Biologics
Pharmaceuticals are typically manufactured through chemical synthesis, involving precise reactions of small molecules under controlled conditions to ensure consistency and purity. Biologics production relies on complex biotechnological processes using living cells or organisms, such as recombinant DNA technology, to produce large, intricate molecules like proteins or antibodies. These manufacturing techniques for biologics require stringent aseptic conditions and advanced bioreactors to maintain cell viability and product stability throughout the process.
Regulatory Pathways for Pharmaceuticals and Biologics
Pharmaceuticals follow a regulatory pathway primarily managed by the FDA's Center for Drug Evaluation and Research (CDER), requiring extensive preclinical studies and phased clinical trials to establish safety and efficacy. Biologics are regulated by the FDA's Center for Biologics Evaluation and Research (CBER) or sometimes CDER, using a Biologics License Application (BLA) process focused on demonstrating product consistency and immunogenicity alongside clinical benefits. Both pathways emphasize rigorous manufacturing standards under Good Manufacturing Practices (GMP), but biologics face additional complexities due to their molecular complexity and sensitivity to production changes.
Market Trends: Pharmaceuticals vs Biologics
The global market for biologics is expanding at a faster rate than traditional pharmaceuticals, driven by increasing demand for targeted therapies in chronic diseases like cancer and autoimmune disorders. Pharmaceuticals still dominate in volume due to their established manufacturing processes and lower production costs, but biologics contribute a higher revenue share because of their complex, high-value nature. Emerging markets are increasingly adopting biologics, supported by advancements in biotechnology and regulatory frameworks facilitating biosimilar approvals, influencing future investment and development trends.
Safety and Efficacy Profiles Compared
Pharmaceuticals and biologics differ significantly in their safety and efficacy profiles due to their distinct manufacturing processes and molecular complexities. Traditional pharmaceuticals, typically small-molecule drugs, have well-established safety records and predictable pharmacokinetics, while biologics, derived from living organisms, may present immunogenic risks but offer targeted efficacy for complex diseases. Regulatory agencies require rigorous clinical trials to evaluate both classes, ensuring that biologics undergo thorough immunogenicity assessments alongside efficacy benchmarks to maintain patient safety.
Pricing and Accessibility Challenges
Pharmaceuticals often have lower production costs compared to biologics, leading to generally more affordable pricing and wider accessibility. Biologics involve complex manufacturing processes and stringent storage requirements, which contribute to significantly higher prices and limited availability, especially in low-income regions. These pricing barriers hinder patient access to cutting-edge treatments, exacerbating disparities in healthcare outcomes globally.
Impact on Patient Outcomes
Pharmaceuticals and biologics differ in their impact on patient outcomes due to their distinct mechanisms of action and production processes. Pharmaceuticals, often small-molecule drugs, primarily target symptoms or disease pathways, providing broad treatment options but sometimes limited specificity. Biologics, derived from living cells, offer targeted therapies that can improve efficacy and reduce side effects, especially in conditions like autoimmune diseases and cancer, enhancing personalized medicine approaches.
Future Directions in Drug Innovation
Pharmaceuticals, primarily small-molecule drugs, are increasingly integrated with biologics to enhance targeted therapies and personalized medicine. Advances in gene editing, monoclonal antibodies, and cell-based therapies are driving the future of biologics, offering novel treatments for complex diseases such as cancer and autoimmune disorders. Emerging trends emphasize combination therapies and precision medicine, leveraging big data and AI to optimize drug efficacy and minimize adverse effects.
Related Important Terms
Biosimilars
Biosimilars are highly similar versions of approved biologics, offering comparable efficacy, safety, and quality at reduced costs, enhancing accessibility in pharmaceutical treatments. Regulatory frameworks demand rigorous analytical and clinical assessments to ensure biosimilars match their reference biologics, positioning them as key drivers in expanding affordable healthcare options globally.
Biobetters
Biobetters, engineered versions of original biologics, offer enhanced efficacy, improved safety profiles, and optimized dosing regimens compared to traditional pharmaceuticals and first-generation biologics. Their targeted molecular design leverages advanced biotechnology, positioning biobetters as key innovations in personalized medicine and chronic disease management.
Small Molecule Drugs
Small molecule drugs, typically synthesized through chemical processes, offer ease of manufacturing, stability, and oral bioavailability, distinguishing them from biologics which are complex, protein-based therapies derived from living cells. These pharmaceuticals target intracellular components, enabling efficient penetration and modulation of specific biochemical pathways, crucial for treating a wide range of diseases including infections, cancer, and chronic conditions.
Cell Therapies
Cell therapies, a subset of biologics, utilize living cells to repair or replace damaged tissues, offering targeted treatment options distinct from traditional pharmaceuticals that rely on chemical compounds. These therapies leverage the regenerative potential of stem cells or immune cells, providing personalized medicine approaches critical for addressing complex diseases like cancer and autoimmune disorders.
Orphan Biologics
Orphan biologics, specialized treatments derived from living organisms, target rare diseases often overlooked by traditional pharmaceuticals due to limited market demand and complex development processes. These biologics offer innovative therapeutic options with higher specificity and efficacy for rare conditions, supported by regulatory incentives like orphan drug status to encourage research and development.
Digital Biologics
Digital biologics integrate advanced digital technologies with traditional biologic therapies to improve precision in drug delivery and personalized treatment outcomes. Unlike conventional pharmaceuticals, digital biologics leverage biosensors and data analytics to monitor patient responses in real-time, enhancing therapeutic efficacy and safety.
Monoclonal Antibodies (mAbs)
Monoclonal antibodies (mAbs), a key class of biologics, are engineered proteins that target specific antigens for treating diseases such as cancer and autoimmune disorders, differing fundamentally from traditional pharmaceuticals which are typically small-molecule drugs synthesized chemically. Their complex structure and mechanism of action enable precise immune modulation, driving advances in personalized medicine and offering therapeutic specificity unavailable with conventional drugs.
RNA Therapeutics
RNA therapeutics represent a cutting-edge class of biologics that utilize messenger RNA (mRNA) to instruct cells in producing specific proteins, contrasting with traditional small-molecule pharmaceuticals that typically target enzymes or receptors. This innovative approach offers promising treatments for genetic disorders, infectious diseases, and cancers by harnessing the body's natural molecular mechanisms for targeted and personalized therapy.
Advanced Therapy Medicinal Products (ATMPs)
Advanced Therapy Medicinal Products (ATMPs) represent a cutting-edge category within pharmaceuticals and biologics, encompassing gene therapies, somatic-cell therapies, and tissue-engineered products designed to treat or cure complex diseases at a molecular or cellular level. Unlike traditional pharmaceuticals, ATMPs utilize living cells or genetic material, offering personalized treatment approaches that address unmet medical needs in conditions such as cancer, genetic disorders, and degenerative diseases.
Targeted Protein Degraders
Targeted protein degraders represent a novel class of therapeutics that differ significantly from traditional pharmaceuticals and biologics by harnessing the cell's natural protein degradation pathways to selectively eliminate disease-causing proteins. These molecules, such as PROTACs (Proteolysis Targeting Chimeras), offer enhanced specificity and efficacy in treating complex diseases like cancer and neurodegenerative disorders by overcoming limitations of conventional drugs in targeting "undruggable" proteins.
Pharmaceuticals vs Biologics Infographic
 industrydif.com
industrydif.com