Clinical trials traditionally require participants to visit centralized locations, which can limit accessibility and slow recruitment. Decentralized trials leverage digital technologies and remote monitoring to enable participation from any location, enhancing patient convenience and accelerating data collection. This approach often increases patient diversity and retention while reducing overall trial costs.
Table of Comparison
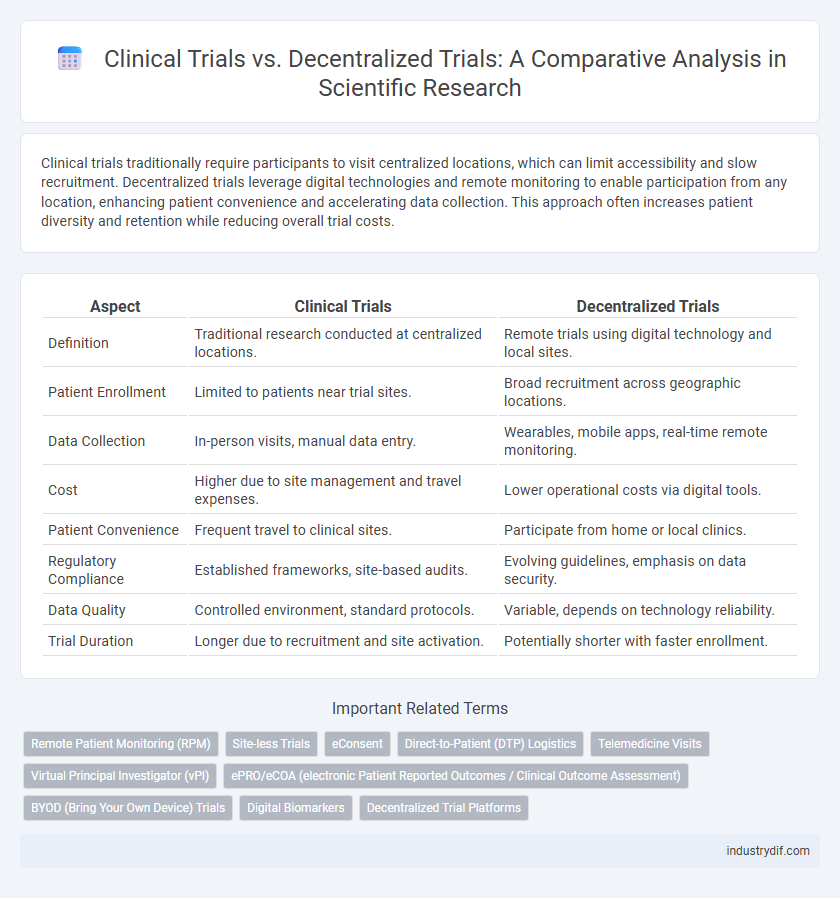
| Aspect | Clinical Trials | Decentralized Trials |
|---|---|---|
| Definition | Traditional research conducted at centralized locations. | Remote trials using digital technology and local sites. |
| Patient Enrollment | Limited to patients near trial sites. | Broad recruitment across geographic locations. |
| Data Collection | In-person visits, manual data entry. | Wearables, mobile apps, real-time remote monitoring. |
| Cost | Higher due to site management and travel expenses. | Lower operational costs via digital tools. |
| Patient Convenience | Frequent travel to clinical sites. | Participate from home or local clinics. |
| Regulatory Compliance | Established frameworks, site-based audits. | Evolving guidelines, emphasis on data security. |
| Data Quality | Controlled environment, standard protocols. | Variable, depends on technology reliability. |
| Trial Duration | Longer due to recruitment and site activation. | Potentially shorter with faster enrollment. |
Definition and Overview of Clinical Trials
Clinical trials are structured research studies conducted to evaluate the safety and effectiveness of new medical interventions, including drugs, devices, or treatment protocols, under controlled conditions. They typically involve multiple phases, from initial safety assessments (Phase I) to large-scale efficacy studies (Phase III) ensuring comprehensive data collection. Traditional clinical trials often require participants to visit centralized research facilities, leading to logistical challenges that decentralized trials seek to address.
Decentralized Trials: Concept and Evolution
Decentralized trials leverage digital technologies such as telemedicine, wearable devices, and mobile apps to conduct clinical research remotely, improving patient accessibility and data collection efficiency. This approach reduces geographical and logistical barriers, enabling broader participant diversity and real-time monitoring while maintaining regulatory compliance through secure data management. Evolving from traditional centralized models, decentralized trials represent a paradigm shift that enhances patient-centricity and accelerates drug development processes.
Key Differences Between Traditional and Decentralized Trials
Traditional clinical trials are conducted at centralized sites with in-person visits, limiting patient diversity and increasing logistical burdens. Decentralized trials leverage digital technologies and remote monitoring to enable patient participation from diverse geographic locations, enhancing enrollment and retention rates. Key differences include data collection methods, patient accessibility, and trial management efficiency, with decentralized trials fostering real-time data integration and patient-centric approaches.
Patient Recruitment and Engagement Strategies
Clinical trials traditionally rely on centralized recruitment methods, often facing challenges in patient accessibility and diversity due to geographic and logistical constraints. Decentralized trials leverage digital tools and remote monitoring, enhancing patient engagement by offering greater convenience and real-time communication, which improves retention rates. Integrated strategies combining virtual screening, e-consent, and wearable technology enable more efficient recruitment and personalized patient support throughout the trial process.
Data Collection Methods in Both Trial Models
Clinical trials traditionally rely on centralized data collection methods involving site visits where patient data is recorded and monitored by clinical staff using electronic data capture (EDC) systems. Decentralized trials utilize remote monitoring technologies such as wearable devices, mobile apps, and telemedicine platforms to collect real-time patient data, increasing data accuracy and patient compliance. These advanced digital tools enable continuous data streams, reducing delays and improving overall trial efficiency compared to conventional site-based methods.
Regulatory Considerations and Compliance
Regulatory considerations in clinical trials emphasize strict adherence to protocols established by agencies such as the FDA and EMA, ensuring data integrity and patient safety throughout the study. Decentralized trials introduce complexities in compliance due to varied regional regulations, remote patient monitoring, and electronic data capture, necessitating robust digital infrastructure and standardized processes. Ensuring harmonization with Good Clinical Practice (GCP) guidelines and maintaining audit trails are critical for both trial models to meet regulatory standards and facilitate successful approvals.
Technological Integration in Decentralized Trials
Technological integration in decentralized trials enhances patient recruitment, data collection, and monitoring through digital platforms such as electronic patient-reported outcomes (ePRO) and wearable devices. Advanced telemedicine tools enable remote consultations and real-time data transmission, reducing the need for physical site visits and improving patient adherence. Cloud-based data management systems facilitate secure, scalable storage and streamlined analysis of clinical trial data, driving efficiency and accuracy in decentralized trial operations.
Challenges and Limitations in Each Trial Type
Clinical trials often face challenges such as high costs, prolonged timelines, and limited patient diversity due to centralized site-based recruitment. Decentralized trials encounter limitations like technology access disparities, data security concerns, and regulatory complexities across different jurisdictions. Both trial types must address patient adherence and data reliability to ensure valid and generalizable outcomes.
Impact on Clinical Outcomes and Data Quality
Decentralized trials enhance clinical outcomes by enabling real-time patient monitoring and improving adherence through remote data collection, which reduces data gaps compared to traditional clinical trials. The integration of digital health technologies in decentralized trials ensures higher data accuracy and timeliness, minimizing variability and bias inherent in centralized site visits. Consequently, decentralized trials contribute to more robust data quality and potentially faster, more reliable assessment of treatment efficacy.
Future Trends in Clinical and Decentralized Trial Designs
Future trends in clinical and decentralized trial designs emphasize hybrid models integrating digital technologies with traditional protocols to enhance patient recruitment and data accuracy. Advanced wearable devices and real-time telemedicine consultations are expected to increase trial accessibility and improve monitoring precision. Artificial intelligence-driven analytics will streamline data management and optimize trial outcomes by predicting patient responses more effectively.
Related Important Terms
Remote Patient Monitoring (RPM)
Remote Patient Monitoring (RPM) in decentralized clinical trials enhances data accuracy and patient adherence by enabling continuous real-time health tracking outside traditional clinical settings, reducing the need for frequent site visits. RPM technology facilitates timely intervention and personalized treatment adjustments, improving overall trial efficiency and patient engagement compared to conventional clinical trials.
Site-less Trials
Site-less trials, a subset of decentralized clinical trials, eliminate the need for traditional physical locations by leveraging telemedicine, mobile health technologies, and direct-to-patient drug delivery. This approach enhances patient recruitment and retention, reduces logistical costs, and accelerates data collection while maintaining regulatory compliance and data integrity.
eConsent
eConsent in decentralized trials enhances patient engagement and streamlines informed consent by enabling remote, interactive, and real-time document review via digital platforms, improving data accuracy and compliance. Traditional clinical trials rely on in-person consent processes, which can limit participant diversity and increase logistical challenges, whereas decentralized trials leverage eConsent to facilitate broader access and faster enrollment.
Direct-to-Patient (DTP) Logistics
Direct-to-Patient (DTP) logistics in decentralized trials streamline medication delivery and sample collection directly to patients' homes, reducing the need for site visits and enhancing patient retention. Traditional clinical trials rely heavily on centralized sites for drug distribution and monitoring, which can delay treatment initiation and limit patient access.
Telemedicine Visits
Telemedicine visits in decentralized trials enhance patient accessibility by enabling remote monitoring and real-time data collection, reducing geographic and logistic barriers common in traditional clinical trials. This integration improves patient adherence and diversity in study populations while maintaining rigorous data quality and regulatory compliance.
Virtual Principal Investigator (vPI)
Virtual Principal Investigator (vPI) platforms enhance decentralized clinical trials by enabling remote oversight, real-time data monitoring, and improved patient engagement, which accelerates study timelines and reduces operational costs. Integrating vPI technologies supports compliance with regulatory standards while maintaining data integrity and fostering participant safety in virtual trial environments.
ePRO/eCOA (electronic Patient Reported Outcomes / Clinical Outcome Assessment)
ePRO/eCOA systems in decentralized trials enhance real-time patient data capture and improve compliance compared to traditional clinical trials, offering greater flexibility and reducing site burden. This digital approach facilitates remote monitoring and accelerates data collection, thereby increasing the reliability and efficiency of outcome assessments in diverse patient populations.
BYOD (Bring Your Own Device) Trials
BYOD trials in decentralized clinical studies leverage participants' personal devices to increase data accuracy and real-time monitoring, reducing site visits and operational costs. This approach enhances patient engagement and trial accessibility while maintaining regulatory compliance through secure data integration and standardized protocols.
Digital Biomarkers
Digital biomarkers in decentralized trials enable real-time, continuous data collection through wearable sensors and mobile devices, enhancing patient monitoring compared to traditional clinical trials. This shift allows for increased patient engagement, reduced geographic barriers, and more diverse data sets, accelerating drug development and personalized medicine.
Decentralized Trial Platforms
Decentralized trial platforms leverage digital technologies such as telemedicine, remote patient monitoring, and electronic data capture to enhance participant recruitment, retention, and real-time data collection, addressing limitations of traditional clinical trials. These platforms facilitate broader geographic reach, reduce costs, and improve patient-centricity by enabling trials to be conducted outside conventional clinical settings.
Clinical Trials vs Decentralized Trials Infographic
 industrydif.com
industrydif.com