Double-blind studies prevent bias by ensuring neither participants nor researchers know who receives the treatment, enhancing objectivity in scientific pet research. Triple-blind studies extend this by also keeping data analysts unaware of group assignments, further minimizing bias in outcome interpretation. This rigorous approach improves the reliability of results in evaluating pet health interventions.
Table of Comparison
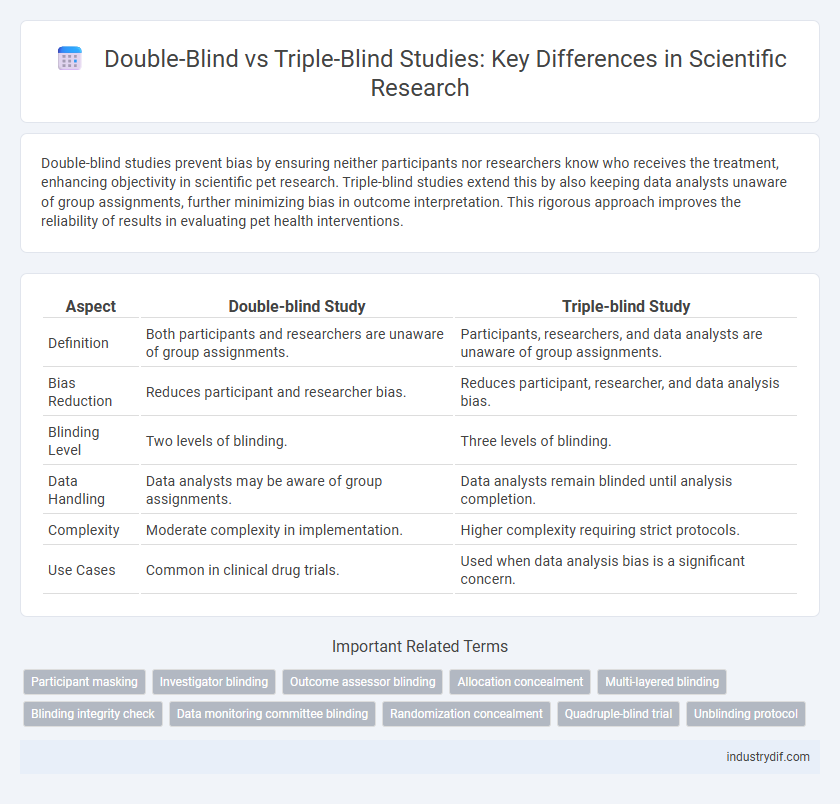
| Aspect | Double-blind Study | Triple-blind Study |
|---|---|---|
| Definition | Both participants and researchers are unaware of group assignments. | Participants, researchers, and data analysts are unaware of group assignments. |
| Bias Reduction | Reduces participant and researcher bias. | Reduces participant, researcher, and data analysis bias. |
| Blinding Level | Two levels of blinding. | Three levels of blinding. |
| Data Handling | Data analysts may be aware of group assignments. | Data analysts remain blinded until analysis completion. |
| Complexity | Moderate complexity in implementation. | Higher complexity requiring strict protocols. |
| Use Cases | Common in clinical drug trials. | Used when data analysis bias is a significant concern. |
Introduction to Blinding in Scientific Research
Blinding in scientific research minimizes bias by concealing group assignments from participants and researchers, enhancing the validity of experimental results. A double-blind study ensures that neither participants nor experimenters know the treatment allocations, reducing placebo effects and observer bias. Triple-blind studies extend this concealment to data analysts, further securing impartiality during data interpretation and reporting.
Defining Double-Blind Studies
Double-blind studies are experimental designs in which both the participants and the researchers administering the treatment are unaware of the group assignments, minimizing bias in data collection and interpretation. This methodology enhances the validity of results by preventing placebo effects and observer bias, particularly in clinical trials testing new drugs or interventions. In contrast, triple-blind studies extend this blinding to data analysts, further reducing potential sources of bias during data processing and statistical analysis.
Defining Triple-Blind Studies
Triple-blind studies extend the blinding process by concealing intervention allocation from participants, researchers administering the treatment, and the data analysts evaluating the results, thereby reducing bias at multiple levels. This methodology enhances the objectivity and reliability of the findings compared to double-blind studies, where only participants and administering researchers are blinded. Implementing triple-blinding is especially crucial in clinical trials where minimizing unconscious bias can significantly impact the validity of outcome measures and data interpretation.
Key Differences Between Double-Blind and Triple-Blind Methods
Double-blind studies conceal group assignments from both participants and researchers conducting assessments, reducing biases related to placebo effects and observer influence. Triple-blind studies extend this by also blinding data analysts, minimizing bias during data interpretation and enhancing result objectivity. The additional blinding layer in triple-blind designs strengthens the study's internal validity by preventing conscious or unconscious manipulation of data outcomes.
Scientific Rationale for Blinding Techniques
Blinding techniques in clinical trials minimize bias by preventing participants, researchers, or analysts from knowing treatment assignments, thus ensuring objective assessment of outcomes. Double-blind studies conceal allocation from both participants and investigators, reducing placebo effects and observer bias, whereas triple-blind studies extend this by also blinding data analysts, further safeguarding against analytic bias and enhancing result validity. The scientific rationale centers on eliminating subjective influences at multiple levels of the study process to produce more reliable and reproducible evidence.
Applications in Clinical Trials
Double-blind studies, where both participants and researchers are unaware of treatment allocation, minimize bias in clinical trials evaluating drug efficacy and safety. Triple-blind studies extend this blinding to data analysts, further reducing bias during data interpretation and enhancing the reliability of results in complex trials with subjective outcome measures. These methodologies are crucial in preventing placebo effects, measurement bias, and confirmation bias, thereby increasing the validity and credibility of clinical trial outcomes.
Advantages of Double-Blind Studies
Double-blind studies reduce bias by preventing both participants and researchers from knowing who receives the treatment or placebo, enhancing the objectivity and reliability of the results. This design minimizes placebo effects and observer bias more efficiently than single-blind studies, maintaining high internal validity. Compared to triple-blind studies, double-blind trials are often easier to implement while still providing robust control over experimental bias.
Advantages of Triple-Blind Studies
Triple-blind studies enhance research validity by eliminating bias from participants, researchers, and data analysts, thereby increasing the objectivity of results. This methodology reduces placebo effects and observer bias more effectively than double-blind studies, leading to more reliable and reproducible outcomes. Incorporating an additional layer of blinding minimizes conscious or unconscious influence on data interpretation, crucial for high-stakes scientific research.
Common Limitations and Challenges
Double-blind and triple-blind studies both face challenges such as increased complexity in maintaining blinding, which can lead to operational difficulties and inadvertent bias. Common limitations include potential unblinding due to side effects or study design flaws, compromising the integrity of data collection and analysis. Ensuring strict adherence to protocols while minimizing human error remains a critical concern impacting the reliability of both study types.
Impact on Research Validity and Bias Reduction
Double-blind studies minimize researcher and participant bias by concealing group assignments, enhancing internal validity through objective outcome assessment. Triple-blind studies extend this by also blinding data analysts, further reducing analytic bias and increasing reliability of results. This additional layer of blinding strengthens evidence credibility and limits potential confounding influences in experimental research.
Related Important Terms
Participant masking
In double-blind studies, both participants and researchers administering the treatment are unaware of group assignments, minimizing bias during data collection; in triple-blind studies, an additional layer of masking extends to data analysts or other personnel involved in data interpretation, further reducing bias in outcome assessment. Participant masking in both designs is crucial to prevent placebo effects and maintain the integrity of experimental results in clinical trials.
Investigator blinding
In a double-blind study, both participants and investigators are unaware of the treatment allocation, minimizing bias in data collection and assessment. A triple-blind study extends this blinding to include data analysts or other key personnel, further reducing bias during data interpretation and ensuring objective results.
Outcome assessor blinding
Outcome assessor blinding in double-blind studies limits bias by preventing assessors from knowing participant allocations, enhancing result objectivity. Triple-blind studies extend this by also masking data analysts, further reducing bias and improving the validity of outcome measurements.
Allocation concealment
Double-blind studies involve both participants and researchers being unaware of group assignments, minimizing performance and detection biases, while triple-blind studies extend this concealment to data analysts, further reducing potential bias during data interpretation. Allocation concealment in both designs ensures that the process of assigning participants to treatment or control groups remains hidden prior to allocation, preventing selection bias and safeguarding internal validity.
Multi-layered blinding
Multi-layered blinding in double-blind studies ensures both participants and researchers administering interventions remain unaware of group assignments, reducing bias in outcome assessment. Triple-blind studies extend this by additionally blinding data analysts, further minimizing potential bias throughout data interpretation and enhancing the study's internal validity.
Blinding integrity check
Blinding integrity checks in double-blind studies involve assessing whether participants and researchers remain unaware of treatment assignments, reducing bias in outcome evaluation. In contrast, triple-blind studies extend this process by including statisticians or data analysts in the blinding, enhancing objectivity through additional layers of concealment and rigorous validation of blinding efficacy.
Data monitoring committee blinding
In double-blind studies, the data monitoring committee typically remains unblinded to ensure unbiased oversight of safety and efficacy data, while in triple-blind studies, the committee is also blinded to prevent bias during interim analyses, enhancing the integrity of data evaluation. Maintaining blinding within the data monitoring committee minimizes the risk of conscious or unconscious influence on trial outcomes and decisions.
Randomization concealment
Randomization concealment in double-blind studies prevents participants and experimenters from knowing group allocations, reducing selection bias, while triple-blind studies extend this concealment to data analysts, further minimizing bias during data interpretation. Ensuring thorough randomization concealment enhances the validity and reliability of clinical trial outcomes by maintaining impartiality throughout study execution and analysis.
Quadruple-blind trial
A quadruple-blind trial extends the blinding process by ensuring that participants, investigators, outcome assessors, and data analysts remain unaware of group assignments, minimizing bias more effectively than double-blind and triple-blind studies. This rigorous methodology enhances the reliability and validity of scientific results by preventing conscious and unconscious influence across multiple levels of the research process.
Unblinding protocol
Double-blind studies prevent both participants and researchers from knowing the treatment allocation, reducing bias, while triple-blind studies extend this by also masking data analysts or outcome assessors, enhancing objectivity. Unblinding protocols in triple-blind studies require stringent controls and predefined criteria to ensure that revealing group assignments occurs only when necessary for safety or regulatory compliance, minimizing premature disclosure and preserving study integrity.
Double-blind Study vs Triple-blind Study Infographic
 industrydif.com
industrydif.com