PCR amplifies DNA sequences to detect the presence of genetic material, providing relative quantification based on amplification cycles, while Digital PCR partitions samples into thousands of micro-reactions enabling absolute quantification with higher sensitivity and precision. Digital PCR excels in detecting low-abundance targets and rare mutations due to its ability to count individual DNA molecules without standard curves. This makes Digital PCR particularly useful for applications requiring accurate quantification in complex samples, such as oncology and infectious disease diagnostics.
Table of Comparison
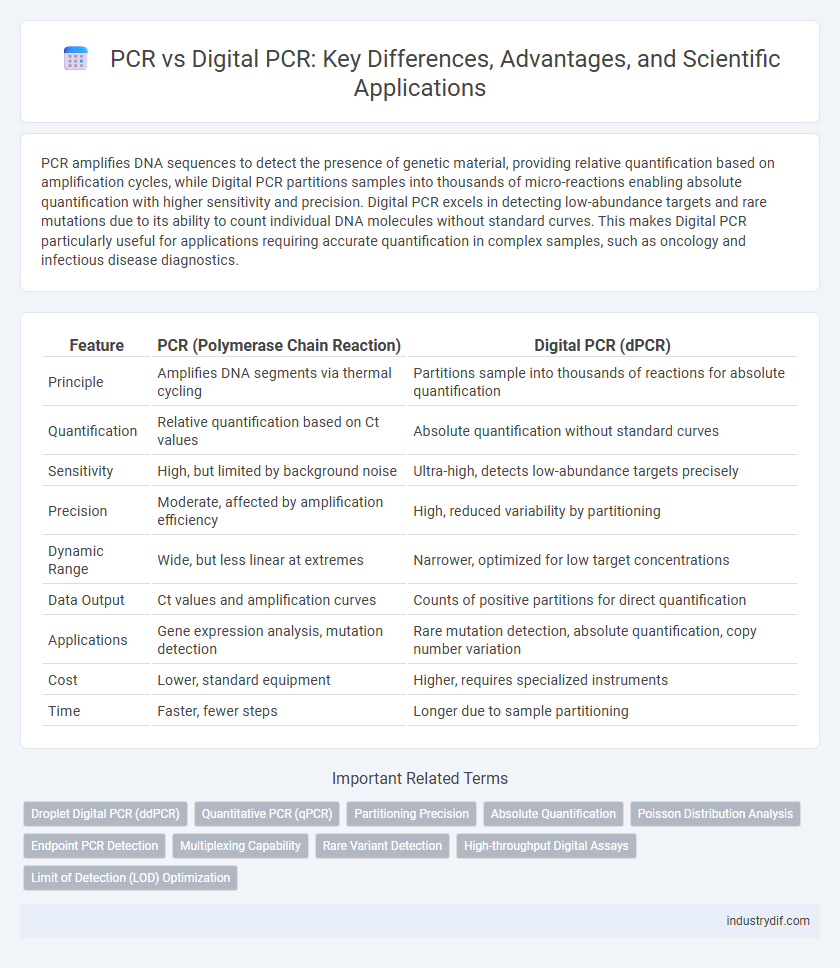
| Feature | PCR (Polymerase Chain Reaction) | Digital PCR (dPCR) |
|---|---|---|
| Principle | Amplifies DNA segments via thermal cycling | Partitions sample into thousands of reactions for absolute quantification |
| Quantification | Relative quantification based on Ct values | Absolute quantification without standard curves |
| Sensitivity | High, but limited by background noise | Ultra-high, detects low-abundance targets precisely |
| Precision | Moderate, affected by amplification efficiency | High, reduced variability by partitioning |
| Dynamic Range | Wide, but less linear at extremes | Narrower, optimized for low target concentrations |
| Data Output | Ct values and amplification curves | Counts of positive partitions for direct quantification |
| Applications | Gene expression analysis, mutation detection | Rare mutation detection, absolute quantification, copy number variation |
| Cost | Lower, standard equipment | Higher, requires specialized instruments |
| Time | Faster, fewer steps | Longer due to sample partitioning |
Introduction to PCR and Digital PCR
Polymerase Chain Reaction (PCR) is a widely used molecular biology technique that amplifies specific DNA sequences, enabling the detection and analysis of genetic material with high sensitivity. Digital PCR (dPCR) advances traditional PCR by partitioning the sample into thousands of micro-reactions, allowing for absolute quantification of nucleic acids without the need for standard curves. This enhanced precision and sensitivity in dPCR make it particularly valuable for applications such as rare mutation detection and low-abundance target quantification.
Fundamental Principles of PCR
Polymerase Chain Reaction (PCR) amplifies DNA by cycling through denaturation, annealing, and extension phases, enabling exponential replication of target sequences using DNA polymerase enzymes. Digital PCR partitions the sample into thousands of micro-reactions, allowing absolute quantification by counting positive amplifications without relying on standard curves. The fundamental principle of PCR lies in thermal cycling to create millions of DNA copies, while digital PCR enhances sensitivity and precision through sample partitioning and endpoint detection.
Fundamentals of Digital PCR Technology
Digital PCR (dPCR) partitions a DNA sample into thousands of individual reactions, allowing absolute quantification of nucleic acids without relying on standard curves. This technology employs fluorescence-based detection to count positive partitions, enhancing sensitivity and precision, especially for low-abundance targets or rare mutations. Compared to conventional PCR, dPCR provides improved accuracy by reducing amplification bias and enabling direct measurement of target molecules.
Key Differences: PCR vs Digital PCR
PCR (Polymerase Chain Reaction) amplifies DNA sequences exponentially, relying on fluorescence signals measured at the end of the cycles to provide relative quantification, while Digital PCR partitions the sample into thousands of micro-reactions, enabling absolute quantification by counting positive partitions. Digital PCR offers higher sensitivity and precision, detecting low-abundance targets and minor genetic variations with improved reproducibility compared to traditional PCR. The main distinction lies in Digital PCR's ability to perform absolute quantification without the need for calibration curves, making it a powerful tool for detecting rare mutations and copy number variations.
Sensitivity and Accuracy Comparison
Digital PCR (dPCR) offers significantly higher sensitivity and accuracy than traditional PCR by partitioning the sample into thousands of individual reactions, enabling precise quantification of low-abundance nucleic acids. This partitioning reduces amplification bias and enhances the detection of rare mutations or low-level pathogens, outperforming conventional PCR's limit of detection. Quantitative data from dPCR demonstrate lower variability and improved reproducibility, making it the preferred method for applications requiring precise nucleic acid quantification.
Quantification Capabilities: Absolute vs Relative
Digital PCR (dPCR) provides absolute quantification by partitioning a sample into thousands of individual reactions, allowing for precise molecule counting without the need for standard curves. In contrast, traditional PCR quantification is relative, relying on cycle threshold (Ct) values and external references to infer target concentrations. The enhanced sensitivity and accuracy of dPCR make it superior for detecting low-abundance targets and genetic variations in complex samples.
Applications in Molecular Diagnostics
PCR enables rapid amplification of DNA sequences, making it essential for detecting genetic mutations, infectious agents, and hereditary diseases in molecular diagnostics. Digital PCR provides precise quantification of nucleic acids with higher sensitivity and specificity, allowing detection of low-level mutations, rare pathogens, and minimal residual disease in cancer monitoring. The choice between PCR and digital PCR depends on diagnostic accuracy requirements, sample complexity, and target nucleic acid abundance.
Workflow and Instrumentation Requirements
Digital PCR (dPCR) workflow involves partitioning the sample into thousands of individual reactions, enabling absolute quantification without the need for standard curves, whereas traditional PCR relies on bulk sample amplification and relative quantification. Instrumentation for dPCR requires specialized droplet generators or microfluidic devices to create partitions and high-precision fluorescence readers for endpoint analysis, in contrast to conventional PCR machines that perform thermal cycling and real-time fluorescence detection. The increased complexity and cost of dPCR instrumentation are balanced by its higher sensitivity, precision, and ability to detect low-abundance targets in complex samples.
Cost and Efficiency Considerations
Digital PCR offers higher sensitivity and precision compared to traditional PCR, enhancing detection of low-abundance targets while generally incurring higher costs due to specialized equipment and reagents. Traditional PCR remains cost-effective and faster for routine analyses, though it may lack the quantitative accuracy needed for complex samples. Selecting between PCR and digital PCR depends on balancing budget constraints with the required analytical sensitivity and throughput for specific scientific applications.
Future Trends in PCR Technologies
Emerging trends in PCR technologies emphasize the integration of digital PCR (dPCR) with microfluidics and multiplexing capabilities, enhancing sensitivity and quantification accuracy at ultra-low DNA concentrations. Advances in portable, real-time dPCR platforms are driving point-of-care diagnostics and environmental monitoring, enabling rapid detection of genetic mutations and rare alleles. Future PCR technologies are also leveraging machine learning algorithms to optimize assay design and data analysis, increasing throughput and reducing error rates in clinical and research applications.
Related Important Terms
Droplet Digital PCR (ddPCR)
Droplet Digital PCR (ddPCR) enhances traditional PCR by partitioning samples into thousands of nanoliter-sized droplets, enabling absolute quantification of target DNA without the need for standard curves. This increased sensitivity and precision make ddPCR especially advantageous for detecting low-abundance mutations and quantifying rare nucleic acid sequences in complex samples.
Quantitative PCR (qPCR)
Quantitative PCR (qPCR) offers real-time amplification measurement using fluorescent markers to quantify nucleic acids, providing high sensitivity and a broad dynamic range compared to Digital PCR's absolute quantification through partitioning. While qPCR excels in speed and throughput for gene expression analysis, Digital PCR delivers enhanced precision for low-abundance target detection and rare mutation quantification.
Partitioning Precision
Digital PCR offers superior partitioning precision by dividing the sample into thousands to millions of individual reactions, enabling absolute quantification of target nucleic acids with high sensitivity and specificity. In contrast, traditional PCR relies on bulk amplification, resulting in less precise quantification due to variable amplification efficiency across the sample.
Absolute Quantification
Digital PCR provides absolute quantification by partitioning the sample into thousands of reactions, allowing precise counting of target DNA molecules without the need for standard curves. Conventional PCR relies on relative quantification through fluorescence intensity, which is influenced by amplification efficiency and requires calibration for accurate measurement.
Poisson Distribution Analysis
Digital PCR enhances quantification accuracy by partitioning samples into thousands of micro-reactions, enabling precise molecule counting through Poisson distribution analysis. This statistical approach corrects for the probability of multiple target molecules occupying the same partition, providing absolute quantification unattainable in conventional PCR methods.
Endpoint PCR Detection
Digital PCR provides absolute quantification by partitioning the sample into thousands of individual reactions, enhancing sensitivity and precision in endpoint PCR detection compared to traditional PCR, which relies on relative fluorescence measurements at the end of amplification cycles. This partitioning reduces the impact of inhibitors and enables detection of low-abundance targets with higher accuracy in endpoint analysis.
Multiplexing Capability
Digital PCR exhibits superior multiplexing capability compared to traditional PCR by partitioning samples into thousands of individual reactions, enabling precise quantification of multiple targets simultaneously with increased sensitivity and reduced competition among primers. This enhanced multiplexing power allows for comprehensive genetic analysis in complex samples, critical for applications requiring accurate detection and quantitation of multiple nucleic acid sequences.
Rare Variant Detection
Digital PCR offers superior sensitivity and precision in rare variant detection compared to traditional PCR by partitioning the sample into thousands of individual reactions, enabling absolute quantification of low-frequency mutations. This method reduces background noise and amplification bias, facilitating the accurate identification of rare alleles crucial for applications in cancer research and genetic disease diagnostics.
High-throughput Digital Assays
High-throughput digital PCR offers enhanced sensitivity and absolute quantification by partitioning samples into thousands of nanoliter reactions, surpassing traditional PCR's relative quantification limits. This technique enables precise detection of rare mutations and low-abundance targets, making it invaluable for genomic applications requiring high analytical accuracy and throughput.
Limit of Detection (LOD) Optimization
Digital PCR offers superior Limit of Detection (LOD) optimization compared to conventional PCR by partitioning samples into thousands of micro-reactions, enhancing sensitivity and quantification accuracy for low-abundance nucleic acids. This partitioning minimizes background noise and enables absolute quantification without reliance on standard curves, making digital PCR the preferred method for applications requiring precise detection of rare genetic variants.
PCR vs Digital PCR Infographic
 industrydif.com
industrydif.com