Genomics studies the complete DNA sequence of an organism, revealing inherited genetic information and variations that influence traits and diseases. Epigenomics examines chemical modifications on DNA and histones that regulate gene expression without altering the genetic code, impacting how genes are turned on or off in response to environmental factors. Together, genomics and epigenomics provide a comprehensive understanding of genetic regulation and its role in health and disease.
Table of Comparison
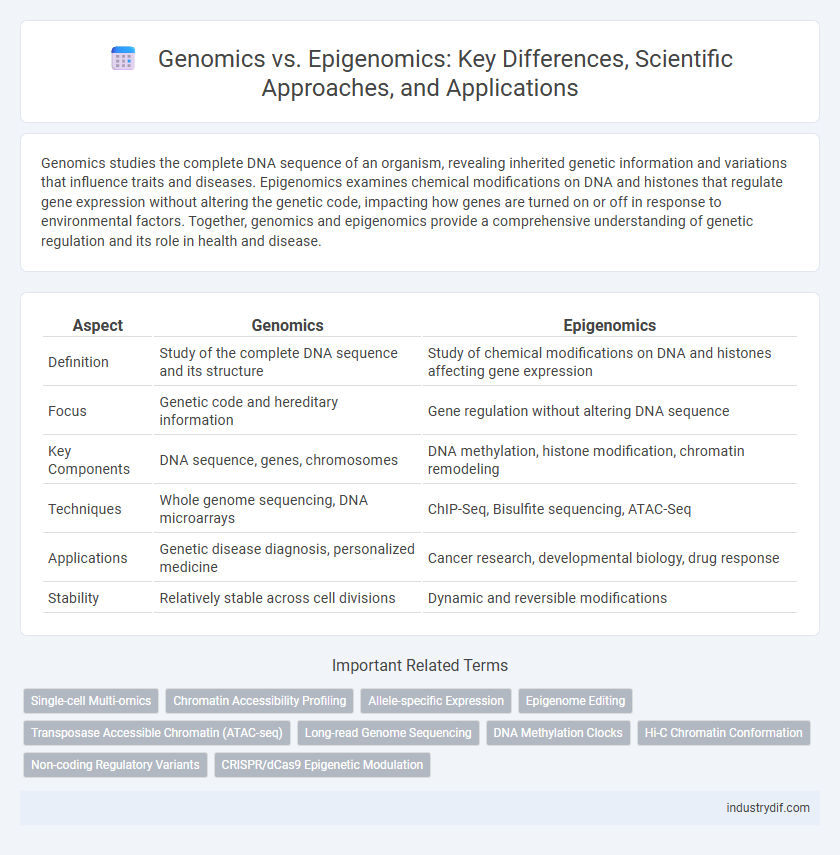
| Aspect | Genomics | Epigenomics |
|---|---|---|
| Definition | Study of the complete DNA sequence and its structure | Study of chemical modifications on DNA and histones affecting gene expression |
| Focus | Genetic code and hereditary information | Gene regulation without altering DNA sequence |
| Key Components | DNA sequence, genes, chromosomes | DNA methylation, histone modification, chromatin remodeling |
| Techniques | Whole genome sequencing, DNA microarrays | ChIP-Seq, Bisulfite sequencing, ATAC-Seq |
| Applications | Genetic disease diagnosis, personalized medicine | Cancer research, developmental biology, drug response |
| Stability | Relatively stable across cell divisions | Dynamic and reversible modifications |
Definition of Genomics
Genomics is the study of an organism's complete set of DNA, including all of its genes, which provides comprehensive insights into genetic sequences, variations, and functions. It involves analyzing the structure, function, evolution, and mapping of genomes to understand inherited traits and disease mechanisms. Techniques such as whole-genome sequencing, genotyping, and bioinformatics are essential tools in genomics research.
Definition of Epigenomics
Epigenomics refers to the study of chemical modifications on the DNA and histone proteins that regulate gene expression without altering the underlying DNA sequence. These modifications, such as DNA methylation and histone acetylation, influence cellular function by turning genes on or off in a dynamic and reversible manner. Unlike genomics, which examines the static genetic code, epigenomics explores the regulatory mechanisms that respond to environmental and developmental cues.
Key Differences Between Genomics and Epigenomics
Genomics studies the entire DNA sequence and its structure, focusing on genetic variations and mutations that influence hereditary traits and diseases. Epigenomics examines chemical modifications on DNA and histone proteins that regulate gene expression without altering the DNA sequence, impacting cell differentiation and environmental responses. Key distinctions involve genomics analyzing static genetic information, while epigenomics investigates dynamic regulatory mechanisms affecting gene activity.
Techniques Used in Genomic Analysis
Genomic analysis primarily utilizes techniques such as whole-genome sequencing, single nucleotide polymorphism (SNP) genotyping, and comparative genomic hybridization (CGH) to identify genetic variations and mutations across DNA sequences. High-throughput sequencing technologies, including next-generation sequencing (NGS), enable detailed examination of genomic structure, function, and genetic diversity. Bioinformatics tools and algorithms are essential for processing large genomic datasets, identifying gene variants, and correlating genetic information with phenotypic traits.
Techniques Used in Epigenomic Analysis
Epigenomic analysis employs techniques such as bisulfite sequencing for DNA methylation profiling, chromatin immunoprecipitation sequencing (ChIP-seq) to map histone modifications, and assay for transposase-accessible chromatin using sequencing (ATAC-seq) to assess chromatin accessibility. These methods enable researchers to investigate epigenetic modifications that influence gene expression without altering the DNA sequence. Advanced techniques like single-cell epigenomics further enhance the resolution for studying cellular heterogeneity in complex tissues.
Role of Genomics in Personalized Medicine
Genomics plays a pivotal role in personalized medicine by enabling the analysis of individual genetic variations to tailor medical treatments and preventive strategies. High-throughput sequencing technologies, such as whole-genome sequencing and SNP genotyping, provide comprehensive data on genetic predispositions and drug response. This genomic information facilitates precision diagnosis, risk assessment, and the development of targeted therapies, advancing patient-specific healthcare outcomes.
Epigenomics and Disease Mechanisms
Epigenomics investigates heritable changes in gene expression that occur without alterations in the DNA sequence, playing a crucial role in understanding disease mechanisms. DNA methylation, histone modifications, and non-coding RNAs regulate gene activity, influencing the onset and progression of complex diseases such as cancer, neurological disorders, and autoimmune conditions. Epigenomic profiling enables the identification of biomarkers and therapeutic targets, offering potential for personalized medicine and novel treatment strategies.
Integration of Genomic and Epigenomic Data
Integration of genomic and epigenomic data enhances understanding of gene regulation by combining DNA sequence information with chemical modifications affecting gene expression. Advanced bioinformatics tools enable correlation of genetic variants with epigenetic markers such as DNA methylation and histone modifications, revealing complex mechanisms driving phenotypic diversity and disease susceptibility. Multi-omics approaches facilitate precision medicine by identifying biomarkers that reflect both genetic predispositions and epigenetic states.
Challenges in Genomics and Epigenomics Research
Genomics research faces challenges such as managing vast datasets from whole-genome sequencing and identifying functional elements within non-coding regions. Epigenomics research contends with the complexity of dynamic epigenetic modifications that vary across cell types and environmental conditions, complicating data interpretation. Both fields require advanced computational tools and integrative approaches to unravel the intricate regulatory networks governing gene expression.
Future Trends in Genomics and Epigenomics
Advancements in single-cell sequencing and CRISPR-based technologies are driving the future trends in genomics and epigenomics, enabling precise mapping of genetic and epigenetic variations at unprecedented resolution. Integration of multi-omics data, including transcriptomics and proteomics, is enhancing the understanding of complex gene regulation mechanisms and disease pathways. Emerging AI-driven analytics and cloud computing platforms are accelerating discoveries in personalized medicine and therapeutic interventions by leveraging large-scale genomics and epigenomics datasets.
Related Important Terms
Single-cell Multi-omics
Single-cell multi-omics integrates genomics and epigenomics to profile DNA sequences alongside chromatin accessibility, DNA methylation, and histone modifications at individual cell resolution, revealing cellular heterogeneity and regulatory mechanisms. This approach enables comprehensive characterization of gene expression regulation and cellular identity in complex tissues, advancing precision medicine and developmental biology.
Chromatin Accessibility Profiling
Chromatin accessibility profiling is a critical technique distinguishing genomics from epigenomics by mapping open chromatin regions to reveal regulatory elements controlling gene expression without altering DNA sequences. Techniques like ATAC-seq and DNase-seq highlight epigenomic landscapes by identifying accessible chromatin that influence cell-type-specific transcriptional regulation.
Allele-specific Expression
Allele-specific expression (ASE) reveals how genetic variants influence gene expression by measuring differences in RNA output between maternal and paternal alleles, highlighting the interplay between genomics and epigenomics. Integrating DNA sequence analysis with epigenetic markers such as DNA methylation and histone modifications allows precise identification of regulatory mechanisms driving ASE in complex traits and diseases.
Epigenome Editing
Epigenome editing involves precise modifications of chemical marks on DNA and histones to regulate gene expression without altering the underlying genetic code, offering potential for targeted therapeutics in diseases such as cancer and neurological disorders. Techniques like CRISPR-dCas9 fused with epigenetic modifiers enable site-specific changes to chromatin structure, advancing personalized medicine by reversing aberrant epigenetic states.
Transposase Accessible Chromatin (ATAC-seq)
ATAC-seq, a high-resolution method for profiling chromatin accessibility, reveals regulatory elements by identifying transposase-accessible regions in both genomics and epigenomics studies. This technique enables the mapping of dynamic epigenomic landscapes, distinguishing open chromatin states that influence gene expression without altering the underlying DNA sequence.
Long-read Genome Sequencing
Long-read genome sequencing provides comprehensive insights into structural variants and complex genomic regions, enhancing the resolution of genomics by accurately mapping DNA sequences over thousands of base pairs. In epigenomics, long-read sequencing enables direct detection of DNA methylation and other epigenetic modifications along individual DNA molecules, offering a more detailed understanding of gene regulation and chromatin states.
DNA Methylation Clocks
DNA methylation clocks, a pivotal tool in epigenomics, measure biological age by quantifying methylation patterns at specific CpG sites across the genome. Unlike genomics, which analyzes static DNA sequences, epigenomics dynamically assesses chemical modifications such as 5-methylcytosine, revealing epigenetic alterations linked to aging and age-related diseases.
Hi-C Chromatin Conformation
Hi-C chromatin conformation captures three-dimensional genome organization by identifying physical interactions between chromatin regions, providing insight beyond DNA sequence variants studied in genomics. Epigenomics leverages Hi-C data to map chromatin architecture and regulatory landscapes, elucidating gene expression control mechanisms influenced by spatial genome folding.
Non-coding Regulatory Variants
Non-coding regulatory variants influence gene expression by altering promoter and enhancer activities without changing the underlying DNA sequence, playing a crucial role in epigenomic mechanisms such as DNA methylation and histone modifications. While genomics identifies these variants through sequence analysis, epigenomics elucidates their functional impact by mapping chromatin accessibility and regulatory element interactions in different cellular contexts.
CRISPR/dCas9 Epigenetic Modulation
CRISPR/dCas9 epigenetic modulation utilizes a catalytically inactive Cas9 protein fused with epigenetic effector domains to specifically alter gene expression without changing the underlying DNA sequence, distinguishing it from traditional genomic editing. This approach enables precise control over histone modifications and DNA methylation patterns, advancing functional genomics and therapeutic interventions in epigenomics.
Genomics vs Epigenomics Infographic
 industrydif.com
industrydif.com