Chemical disinfection effectively eliminates pathogens through the addition of chlorine or chloramine but may produce harmful disinfection byproducts. Ultraviolet advanced oxidation combines UV light with oxidants like hydrogen peroxide to generate reactive radicals that degrade contaminants without residual chemicals. This method enhances water purity by breaking down organic pollutants while minimizing toxic residues.
Table of Comparison
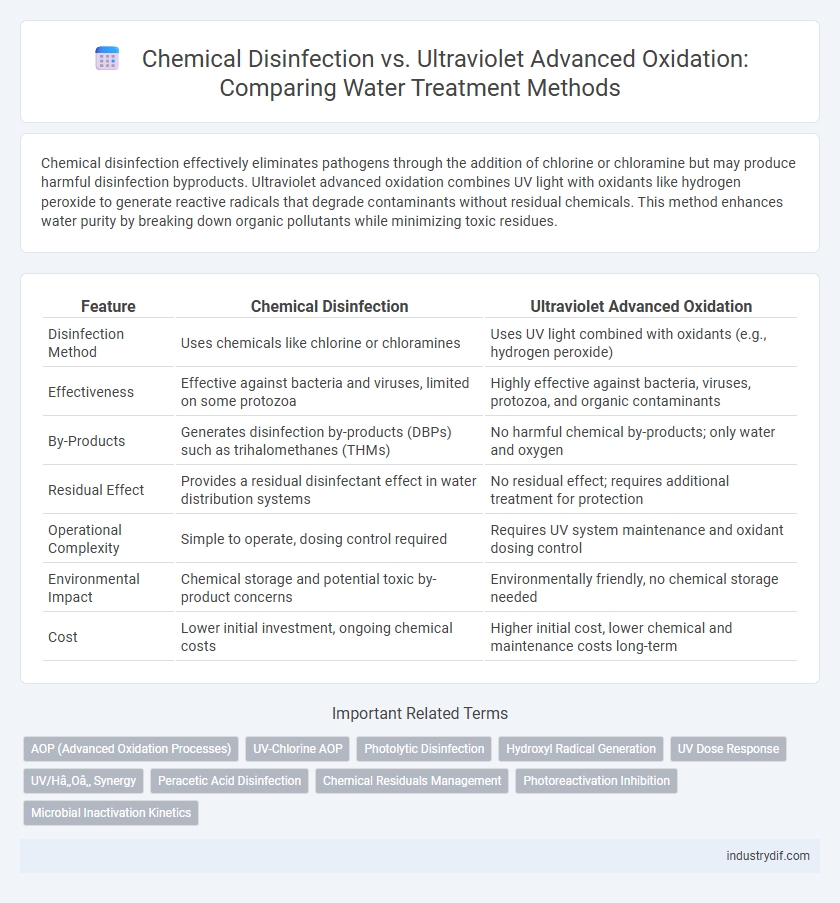
| Feature | Chemical Disinfection | Ultraviolet Advanced Oxidation |
|---|---|---|
| Disinfection Method | Uses chemicals like chlorine or chloramines | Uses UV light combined with oxidants (e.g., hydrogen peroxide) |
| Effectiveness | Effective against bacteria and viruses, limited on some protozoa | Highly effective against bacteria, viruses, protozoa, and organic contaminants |
| By-Products | Generates disinfection by-products (DBPs) such as trihalomethanes (THMs) | No harmful chemical by-products; only water and oxygen |
| Residual Effect | Provides a residual disinfectant effect in water distribution systems | No residual effect; requires additional treatment for protection |
| Operational Complexity | Simple to operate, dosing control required | Requires UV system maintenance and oxidant dosing control |
| Environmental Impact | Chemical storage and potential toxic by-product concerns | Environmentally friendly, no chemical storage needed |
| Cost | Lower initial investment, ongoing chemical costs | Higher initial cost, lower chemical and maintenance costs long-term |
Introduction to Water Disinfection Methods
Chemical disinfection uses agents such as chlorine and chloramine to inactivate pathogens by disrupting cellular functions, providing residual protection in distribution systems. Ultraviolet advanced oxidation generates reactive hydroxyl radicals by combining UV light with oxidants like hydrogen peroxide, effectively degrading contaminants without harmful byproducts. Both methods offer distinct advantages in water treatment, with chemical disinfection ensuring prolonged microbial control and ultraviolet advanced oxidation targeting a broader spectrum of emerging pollutants.
Overview of Chemical Disinfection in Water Treatment
Chemical disinfection in water treatment primarily relies on chlorine, chloramine, or ozone to eliminate pathogens and ensure safe drinking water. This method is widely used due to its residual disinfectant effect, cost-efficiency, and ability to inactivate a broad spectrum of microorganisms. However, chemical disinfection can lead to the formation of disinfection byproducts like trihalomethanes, necessitating careful monitoring and regulation.
Fundamentals of Ultraviolet Advanced Oxidation Processes (AOP)
Ultraviolet Advanced Oxidation Processes (AOP) rely on the generation of highly reactive hydroxyl radicals (*OH) through the photolysis of hydrogen peroxide or ozone under UV radiation, enabling the rapid degradation of organic contaminants and pathogens in water. This method surpasses traditional chemical disinfection by breaking down complex pollutants into harmless end products without forming harmful disinfection by-products (DBPs). The fundamental principle of UV AOP involves synergistic interactions between UV light and oxidants to enhance oxidation efficiency and ensure superior water purification.
Key Mechanisms: Chemical vs UV Advanced Oxidation
Chemical disinfection relies on oxidizing agents like chlorine or chloramine, which disrupt microbial cell walls and denature proteins through oxidative damage. Ultraviolet advanced oxidation combines UV radiation with photocatalysts or oxidants (e.g., hydrogen peroxide) to generate reactive oxygen species such as hydroxyl radicals, leading to comprehensive mineralization of contaminants and pathogen inactivation. The distinct mechanisms highlight chemical disinfection's targeted microbial lysis versus UV advanced oxidation's broad-spectrum degradation of organic pollutants and microorganisms.
Effectiveness Against Microorganisms and Contaminants
Chemical disinfection, such as chlorination, effectively inactivates a broad spectrum of bacteria and viruses but may produce harmful disinfection byproducts and exhibit limited efficacy against certain protozoa and chemical contaminants. Ultraviolet advanced oxidation processes (UV AOP) generate highly reactive hydroxyl radicals that not only inactivate microorganisms, including chlorine-resistant pathogens like Cryptosporidium, but also degrade organic contaminants and micropollutants with high efficiency. UV AOP ensures comprehensive water treatment by combining microbial inactivation and contaminant oxidation without residual chemical risks.
Byproducts and Water Quality Implications
Chemical disinfection processes, such as chlorination, commonly generate disinfection byproducts (DBPs) like trihalomethanes (THMs) and haloacetic acids (HAAs), which pose potential health risks and affect water taste and odor. Ultraviolet advanced oxidation processes (UV-AOPs) significantly reduce the formation of harmful byproducts by producing hydroxyl radicals that degrade organic contaminants without introducing chemical residuals. Water quality under UV-AOP treatment typically shows lower DBP concentrations and improved safety profiles, making it a preferred choice for minimizing toxic byproduct formation in potable water systems.
Operational and Maintenance Considerations
Chemical disinfection requires consistent chemical dosing, regular monitoring of residual chlorine levels, and careful management of chemical storage and handling protocols to ensure safety and efficacy. Ultraviolet advanced oxidation systems demand routine cleaning of UV lamps to prevent fouling, periodic lamp replacements, and continuous monitoring of UV intensity to maintain oxidative performance. Both methods involve distinct operational challenges, with chemical systems emphasizing chemical feed control and UV systems focusing on equipment maintenance and operational parameter stability.
Environmental and Health Impacts
Chemical disinfection methods, such as chlorine treatment, can lead to the formation of harmful disinfection by-products (DBPs) like trihalomethanes, posing risks to human health and aquatic ecosystems. Ultraviolet advanced oxidation processes (UV-AOP) offer a more environmentally friendly alternative by effectively degrading organic contaminants without generating toxic residuals or harmful DBPs. UV-AOP minimizes ecological disruption and reduces potential carcinogenic exposure in drinking water, making it a safer choice for sustainable water treatment.
Cost Comparison and Economic Feasibility
Chemical disinfection typically incurs lower initial capital costs compared to ultraviolet advanced oxidation but demands ongoing expenses for chemical procurement and handling, impacting long-term economic feasibility. Ultraviolet advanced oxidation systems require higher upfront investment in sophisticated equipment and maintenance but benefit from reduced chemical costs and lower secondary pollution management, potentially offering better cost-effectiveness in large-scale or sensitive water treatment applications. Economic feasibility depends on water quality, treatment volume, and regulatory requirements, with ultraviolet advanced oxidation often justified in scenarios prioritizing environmental safety and operational sustainability.
Future Trends in Water Disinfection Technologies
Chemical disinfection methods, such as chlorination, remain widely used due to their cost-effectiveness and residual protection but face challenges related to disinfection by-products and microbial resistance. Ultraviolet (UV) advanced oxidation processes (AOPs), combining UV light with oxidants like hydrogen peroxide, offer enhanced pathogen inactivation and degradation of organic contaminants without harmful residues. Future trends emphasize integrating UV AOPs with real-time monitoring and automation to improve efficiency, sustainability, and adaptability in municipal and industrial water treatment systems.
Related Important Terms
AOP (Advanced Oxidation Processes)
Advanced Oxidation Processes (AOPs) utilize highly reactive hydroxyl radicals to degrade organic contaminants more effectively than traditional chemical disinfection methods, which primarily rely on chlorine or ozone. Ultraviolet AOPs combine UV light with oxidants like hydrogen peroxide to enhance microbial inactivation and reduce harmful disinfection byproducts, offering superior water treatment performance.
UV-Chlorine AOP
UV-Chlorine Advanced Oxidation Process (AOP) effectively combines ultraviolet light and chlorine to generate hydroxyl radicals, offering superior removal of emerging contaminants compared to traditional chemical disinfection. This method enhances water treatment by improving disinfection efficiency while reducing harmful disinfection byproducts such as trihalomethanes and haloacetic acids.
Photolytic Disinfection
Photolytic disinfection leverages ultraviolet (UV) light to generate reactive oxygen species that effectively inactivate pathogens without chemical residuals, ensuring safer water quality. Unlike chemical disinfection methods such as chlorination, photolytic advanced oxidation processes minimize harmful byproducts and reduce the risk of microbial resistance.
Hydroxyl Radical Generation
Chemical disinfection relies on chlorine or ozone to inactivate pathogens, while Ultraviolet Advanced Oxidation processes (UV-AOP) generate hydroxyl radicals (*OH) with higher oxidative potential, enabling more effective degradation of contaminants. Hydroxyl radical generation in UV-AOP enhances water treatment by rapidly breaking down organic pollutants and resistant microorganisms, surpassing traditional chemical disinfectants in both efficiency and byproduct control.
UV Dose Response
Ultraviolet advanced oxidation processes exhibit a more efficient UV dose response compared to traditional chemical disinfection, achieving higher pathogen inactivation rates at lower energy inputs. The UV dose in advanced oxidation generates reactive radicals that not only directly disrupt microbial DNA but also oxidize organic contaminants, enhancing overall water treatment efficacy.
UV/H₂O₂ Synergy
UV/H2O2 advanced oxidation processes generate hydroxyl radicals that effectively degrade persistent organic pollutants beyond the capabilities of chemical disinfection alone. This synergy enhances water treatment efficiency by combining UV photolysis with hydrogen peroxide oxidation, ensuring superior microbial inactivation and contaminant removal in wastewater and drinking water applications.
Peracetic Acid Disinfection
Peracetic acid disinfection offers a powerful chemical alternative to traditional methods, effectively inactivating a wide spectrum of pathogens without forming harmful disinfection byproducts. In comparison to ultraviolet advanced oxidation processes, peracetic acid provides rapid microbial kill rates and maintains residual antimicrobial activity, ensuring ongoing protection in water treatment applications.
Chemical Residuals Management
Chemical disinfection methods often leave harmful residuals such as trihalomethanes (THMs) and chloramines, requiring complex management to prevent water quality degradation and health risks. Ultraviolet advanced oxidation processes generate minimal chemical residuals, significantly reducing the challenges associated with managing disinfection byproducts and improving overall water safety.
Photoreactivation Inhibition
Chemical disinfection effectively kills pathogens but can leave residuals that promote photoreactivation, allowing bacteria to repair UV-induced DNA damage. Ultraviolet advanced oxidation processes (UV-AOP) generate reactive radicals that irreversibly inhibit photoreactivation, ensuring more complete microbial inactivation in water treatment.
Microbial Inactivation Kinetics
Chemical disinfection relies on chlorine and chloramine to achieve rapid microbial inactivation through oxidative damage to cellular components, exhibiting first-order kinetics dependent on disinfectant concentration and contact time. Ultraviolet advanced oxidation processes generate reactive radicals like hydroxyl radicals that degrade microbial DNA and cell walls, resulting in more complex inactivation kinetics often modeled by biphasic or tailing curves.
Chemical Disinfection vs Ultraviolet Advanced Oxidation Infographic
 industrydif.com
industrydif.com