Hard water contains high levels of calcium and magnesium minerals, causing buildup in pipes and reducing soap effectiveness. Soft water, treated to remove these minerals, prevents scale formation and improves cleaning efficiency. Choosing the appropriate water type impacts household appliance longevity and skin health.
Table of Comparison
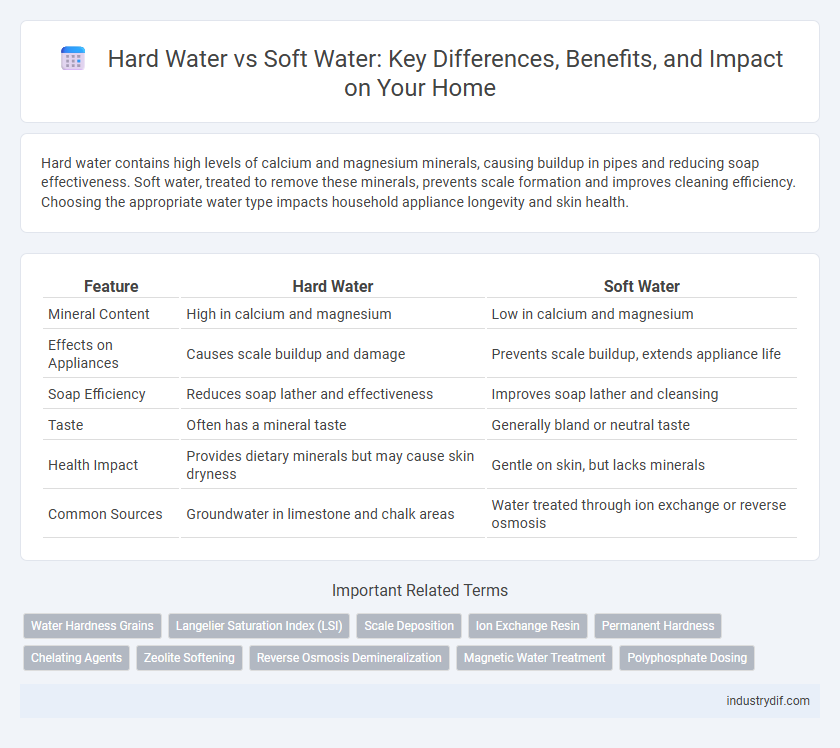
| Feature | Hard Water | Soft Water |
|---|---|---|
| Mineral Content | High in calcium and magnesium | Low in calcium and magnesium |
| Effects on Appliances | Causes scale buildup and damage | Prevents scale buildup, extends appliance life |
| Soap Efficiency | Reduces soap lather and effectiveness | Improves soap lather and cleansing |
| Taste | Often has a mineral taste | Generally bland or neutral taste |
| Health Impact | Provides dietary minerals but may cause skin dryness | Gentle on skin, but lacks minerals |
| Common Sources | Groundwater in limestone and chalk areas | Water treated through ion exchange or reverse osmosis |
Introduction to Hard Water and Soft Water
Hard water contains high concentrations of minerals such as calcium and magnesium ions, which affect its taste, soap lathering, and can cause scale buildup in pipes and appliances. Soft water, in contrast, has low mineral content, making it more effective for cleaning and less likely to leave deposits on surfaces. Understanding the differences in mineral composition between hard and soft water is essential for water treatment and household applications.
Chemical Composition Differences
Hard water contains high concentrations of dissolved minerals, primarily calcium (Ca2+) and magnesium (Mg2+) ions, which contribute to its characteristic hardness. Soft water, in contrast, has low levels of these minerals and often contains higher concentrations of sodium (Na+) or potassium (K+) ions, especially after water softening treatments. The chemical composition difference affects scale formation in pipes and soap scum production during cleaning.
Sources of Hard and Soft Water
Hard water primarily originates from natural sources rich in dissolved minerals, notably calcium and magnesium, which commonly seep through limestone, chalk, and gypsum deposits. Soft water typically comes from precipitation or surface water sources, such as rainwater or rivers, where mineral content is minimal or has been removed through processes like ion exchange or reverse osmosis. Groundwater passing through igneous or volcanic rock formations tends to be soft due to lower mineral dissolution compared to sedimentary rock areas.
Identifying Hard and Soft Water
Hard water contains high levels of calcium and magnesium ions, which cause mineral buildup and reduce soap effectiveness, while soft water has low concentrations of these minerals, making it more efficient for cleaning and bathing. Identifying hard water can be done through visible signs such as soap scum, scale deposits on faucets, and dry skin after washing, whereas soft water produces abundant lather and leaves no residue. Testing kits measuring mineral content or water hardness in grains per gallon (gpg) provide precise identification between hard water, typically above 7 gpg, and soft water below this threshold.
Effects on Plumbing and Appliances
Hard water contains high levels of calcium and magnesium, which cause mineral buildup in pipes and reduce water flow, leading to clogs and increased wear on plumbing systems. Soft water, with minimal mineral content, prevents scale formation, extending the lifespan of appliances such as water heaters, dishwashers, and washing machines. Using soft water reduces maintenance costs and improves appliance efficiency by minimizing corrosion and sediment accumulation.
Impact on Skin and Hair Health
Hard water, rich in minerals like calcium and magnesium, often leaves a residue that can dry out skin and hair, causing irritation and dullness. Soft water, with fewer mineral contents, tends to be gentler, maintaining skin moisture and enhancing hair smoothness and shine. People with sensitive skin or scalp conditions may experience fewer issues when using soft water for washing and bathing.
Soap and Detergent Efficiency
Hard water contains high levels of calcium and magnesium ions that react with soap to form insoluble scum, reducing soap's cleaning effectiveness and leaving residues on surfaces. Soft water, lacking these minerals, allows soap and detergents to lather more easily and rinse away completely, enhancing cleaning efficiency and fabric softness. Detergents formulated with chelating agents perform better in hard water by binding minerals and preventing soap scum formation, but soft water remains optimal for maximum soap and detergent performance.
Scale Buildup and Maintenance Solutions
Hard water contains high levels of calcium and magnesium ions, leading to scale buildup in pipes, appliances, and fixtures that reduces efficiency and increases maintenance costs. Soft water, treated through ion exchange or water softeners, minimizes mineral deposits and prevents scale formation, enhancing the lifespan of plumbing systems and household appliances. Routine descaling treatments and installation of water softening devices are effective maintenance solutions to combat the adverse effects of hard water.
Water Softening Methods and Technologies
Water softening methods primarily include ion exchange, where calcium and magnesium ions are replaced with sodium or potassium ions, effectively reducing hardness. Chelation uses chemical agents to bind hardness minerals, preventing scale formation, while reverse osmosis employs semi-permeable membranes to filter out dissolved minerals and impurities. Advanced technologies like electromagnetic and template-assisted crystallization alter mineral structure to inhibit scale without chemical additives, offering eco-friendly alternatives.
Choosing the Right Water for Industrial Applications
Hard water contains high levels of calcium and magnesium ions, which can lead to scale buildup in industrial equipment, reducing efficiency and increasing maintenance costs. Soft water, treated through ion exchange or reverse osmosis, prevents scaling and corrosion, ensuring optimal performance and longevity of machinery in industries such as manufacturing and food processing. Selecting the right water type depends on specific industrial requirements, balancing cost, equipment sensitivity, and desired operational outcomes.
Related Important Terms
Water Hardness Grains
Water hardness is measured in grains per gallon (gpg), where hard water typically contains more than 7 gpg of dissolved calcium and magnesium ions, causing scale buildup in pipes and appliances. Soft water has less than 1 gpg of these minerals, reducing limescale formation and improving soap efficiency.
Langelier Saturation Index (LSI)
The Langelier Saturation Index (LSI) measures water's tendency to precipitate or dissolve calcium carbonate, indicating whether hard or soft water is more corrosive or scale-forming. Hard water with a positive LSI tends to cause scale buildup, while soft water with a negative LSI can be more corrosive to pipes and fixtures.
Scale Deposition
Hard water contains high levels of calcium and magnesium ions that cause scale deposition on pipes and appliances, reducing their efficiency and lifespan. Soft water, treated to remove these minerals, prevents scale buildup and protects plumbing systems from corrosion and damage.
Ion Exchange Resin
Ion exchange resin effectively softens hard water by replacing calcium and magnesium ions with sodium or potassium ions, reducing scale buildup and improving water quality. This process enhances appliance longevity and increases the efficiency of soaps and detergents in household and industrial applications.
Permanent Hardness
Permanent hardness in water is primarily caused by dissolved calcium and magnesium sulfates or chlorides, which cannot be removed by boiling. This type of hardness requires chemical treatment methods such as ion exchange or the use of water softeners to prevent scale buildup and improve water quality.
Chelating Agents
Chelating agents in hard water bind to calcium and magnesium ions, preventing scale buildup and improving cleaning efficiency in detergents and soaps. Soft water contains fewer minerals, reducing the need for chelating agents and enhancing the performance of cleaning products without causing residue or buildup.
Zeolite Softening
Zeolite softening is an effective ion-exchange process that removes calcium and magnesium ions from hard water, replacing them with sodium ions to produce soft water. This method improves water quality by preventing scale buildup in pipes and appliances, enhancing efficiency and lifespan.
Reverse Osmosis Demineralization
Reverse osmosis effectively removes calcium and magnesium ions from hard water, transforming it into soft water by demineralization, which enhances appliance longevity and improves taste. This filtration process is crucial for reducing scale buildup and preventing damage in plumbing systems.
Magnetic Water Treatment
Magnetic water treatment alters the physical properties of minerals in hard water, reducing scale buildup without chemicals by changing calcium carbonate crystallization. This eco-friendly method is effective in preventing pipe scaling and improving appliance longevity compared to traditional water softening techniques.
Polyphosphate Dosing
Polyphosphate dosing effectively prevents scale buildup in hard water by sequestering calcium and magnesium ions, reducing pipe corrosion and maintaining water system efficiency. In soft water, polyphosphate use is minimal as low mineral content lessens scaling risk, ensuring optimal water quality and equipment longevity.
Hard Water vs Soft Water Infographic
 industrydif.com
industrydif.com