Raw water contains natural levels of deuterium, an isotope of hydrogen, which can influence its physical and chemical properties. Deuterium depleted water (DDW) is specifically processed to reduce deuterium concentration, potentially offering benefits such as improved cellular metabolism and detoxification. The distinction between raw water and DDW is critical for applications requiring precise isotopic composition, including scientific research and health-related uses.
Table of Comparison
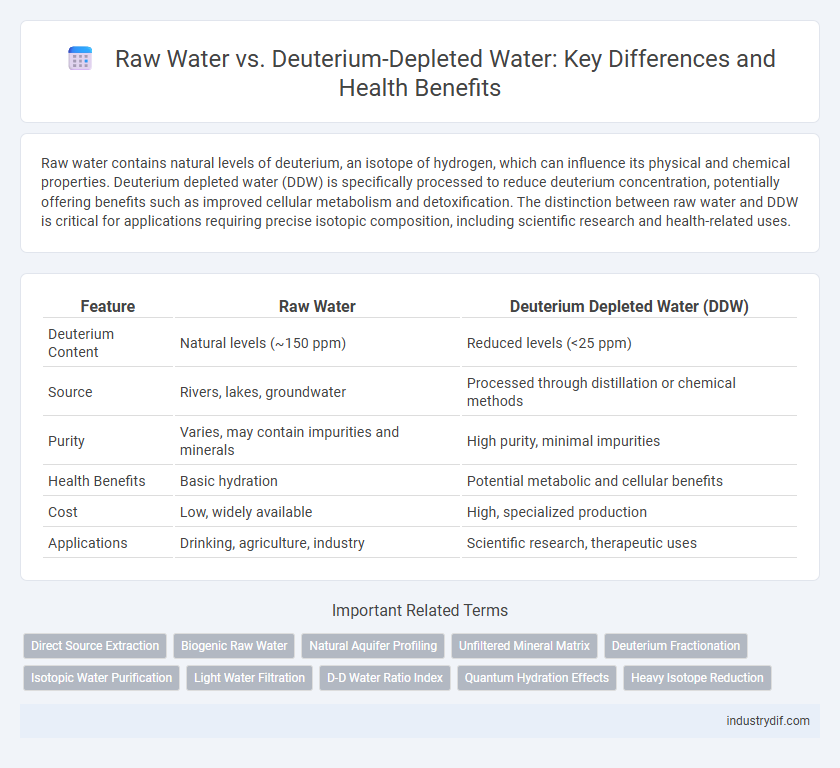
| Feature | Raw Water | Deuterium Depleted Water (DDW) |
|---|---|---|
| Deuterium Content | Natural levels (~150 ppm) | Reduced levels (<25 ppm) |
| Source | Rivers, lakes, groundwater | Processed through distillation or chemical methods |
| Purity | Varies, may contain impurities and minerals | High purity, minimal impurities |
| Health Benefits | Basic hydration | Potential metabolic and cellular benefits |
| Cost | Low, widely available | High, specialized production |
| Applications | Drinking, agriculture, industry | Scientific research, therapeutic uses |
Introduction to Raw Water and Deuterium Depleted Water
Raw water is untreated water sourced directly from natural environments such as rivers, lakes, or underground aquifers, containing various minerals, organic matter, and potentially contaminants. Deuterium depleted water (DDW) undergoes a specialized process to reduce the concentration of deuterium, a heavy isotope of hydrogen, resulting in water with altered isotopic composition and potential health benefits. Understanding the differences in chemical and isotopic content between raw water and DDW is essential for applications in health, research, and industrial processes.
Defining Raw Water: Composition and Sources
Raw water refers to natural, untreated water sourced directly from rivers, lakes, groundwater, or reservoirs, containing various minerals, organic matter, and dissolved gases. Its composition varies widely depending on geographic location, environmental factors, and contamination levels, often including impurities such as sediments, bacteria, and trace metals. This contrasts with deuterium depleted water, which is specifically processed to reduce the concentration of heavy hydrogen isotopes, making raw water a primary, unrefined source before such refinements.
Understanding Deuterium Depleted Water: Characteristics and Production
Deuterium depleted water (DDW) is characterized by a significantly lower concentration of deuterium, a stable hydrogen isotope, compared to raw water, which contains natural levels of deuterium. The production of DDW involves advanced separation techniques such as distillation, electrolysis, or catalytic exchange to reduce deuterium content from approximately 150 ppm found in raw water to levels often below 120 ppm. DDW's altered isotopic composition results in unique physicochemical properties, making it valuable for specialized industrial, medical, and research applications.
Deuterium in Water: Natural Abundance and Industrial Relevance
Deuterium, a stable hydrogen isotope with a natural abundance of about 0.0156%, is present in raw water as heavy water (D2O) and influences its physical and chemical properties. Deuterium-depleted water (DDW) contains lower concentrations of deuterium, typically below 120 ppm, and is engineered through industrial processes such as fractional distillation and electrolysis. The reduction of deuterium in water has significant applications in medical research, nuclear reactors, and agriculture due to its impact on metabolic rates and neutron moderation.
Extraction and Treatment Methods for Raw Water
Extraction of raw water primarily involves sourcing from natural reserves such as rivers, lakes, and underground aquifers using wells, intake pumps, or surface collection. Treatment methods include sedimentation, filtration, and disinfection to remove physical impurities, microorganisms, and chemical contaminants, ensuring safety for consumption or industrial use. Deuterium depleted water undergoes specialized isotopic separation processes, such as fractional distillation or electrolysis, which differ significantly from conventional raw water treatment techniques.
Technologies for Deuterium Depletion in Water
Technologies for deuterium depletion in water primarily include fractional distillation, electrolysis, and catalytic exchange, each leveraging differences in physical and chemical properties between hydrogen isotopes. Fractional distillation exploits the slightly lower boiling point of protium compared to deuterium to separate isotopes effectively. Electrolysis selectively splits water molecules, reducing deuterium content by preferentially decomposing H2O over D2O, while catalytic exchange uses hydrogen isotope exchange reactions facilitated by specialized catalysts to achieve depletion.
Industrial Applications: Raw Water vs Deuterium Depleted Water
Raw water is commonly used in industrial applications such as cooling, boiler feed, and process water, but it often contains impurities that can reduce efficiency and increase maintenance costs. Deuterium depleted water (DDW), with its lower concentration of heavy hydrogen isotopes, is utilized in specialized industries like pharmaceuticals and semiconductor manufacturing where reduced isotope content enhances product quality and chemical reactions. The adoption of DDW in industrial processes can improve reaction kinetics and reduce corrosion, offering cost benefits over conventional raw water despite higher initial processing expenses.
Quality Standards and Regulatory Considerations
Raw water quality is governed by standards set by entities like the EPA, focusing on contaminants, turbidity, and microbial presence to ensure safety for intended use. In contrast, deuterium depleted water (DDW) undergoes specialized processing and must comply with additional purity criteria related to isotopic concentration, often regulated under niche industrial and pharmaceutical guidelines. Regulatory frameworks for DDW emphasize precise isotopic measurement and certification, reflecting its distinct applications compared to conventional raw water.
Health and Environmental Implications
Raw water contains natural isotopic compositions, including standard hydrogen isotopes, whereas deuterium depleted water (DDW) has reduced levels of heavy hydrogen isotope deuterium, which may influence cellular metabolism and mitochondrial function. Studies suggest DDW can potentially improve metabolic health, reduce oxidative stress, and positively affect neuromuscular performance by altering isotopic ratios in biological systems. Environmentally, sourcing DDW involves isotopic separation processes with varying energy requirements, and its consumption could impact natural hydrogen isotope cycles, necessitating evaluation of sustainable production methods and ecological consequences.
Market Trends and Future Prospects
The global market for deuterium depleted water (DDW) is experiencing significant growth driven by increasing applications in pharmaceuticals and biotechnology, where its reduced deuterium content offers enhanced biological effects compared to raw water. Trend analysis indicates a rising demand for DDW in medical research and industrial processes, supported by advancements in isotopic separation technologies and growing awareness of its health benefits. Future prospects point to expanded use in anti-aging and cancer treatment sectors, with market forecasts projecting a compound annual growth rate (CAGR) exceeding 8% over the next decade.
Related Important Terms
Direct Source Extraction
Raw water is sourced directly from natural bodies like rivers, lakes, and underground aquifers, containing a mixture of minerals, organic materials, and varying isotopic compositions. Deuterium Depleted Water (DDW) undergoes specialized extraction and purification processes to reduce deuterium levels below natural abundance, enhancing its use in scientific, medical, and industrial applications.
Biogenic Raw Water
Biogenic raw water contains naturally occurring minerals and trace elements essential for biological processes, contrasting with deuterium depleted water, which has reduced levels of heavy hydrogen isotopes to enhance metabolic efficiency in cells. Studies show that lowering deuterium concentration in water can improve mitochondrial function and may contribute to better energy production and overall cellular health.
Natural Aquifer Profiling
Natural aquifer profiling reveals that raw water typically contains a stable concentration of deuterium, whereas deuterium depleted water (DDW) exhibits significantly lower isotopic ratios, enhancing its potential for specialized industrial and health applications. Understanding isotopic variations in raw aquifer water versus DDW supports precise environmental monitoring and resource management.
Unfiltered Mineral Matrix
Raw water contains a complex unfiltered mineral matrix composed of naturally occurring minerals, trace elements, and organic matter essential for ecological balance and human health. Deuterium Depleted Water (DDW), while similar in mineral content, is specifically processed to reduce deuterium concentration, potentially enhancing metabolic and cellular functions without significantly altering the intrinsic unfiltered mineral matrix.
Deuterium Fractionation
Deuterium fractionation in raw water results from natural isotopic variations influenced by environmental factors such as evaporation and condensation, leading to fluctuating deuterium levels. Deuterium depleted water exhibits a significantly reduced deuterium-to-hydrogen ratio, achieved through advanced separation techniques, enhancing applications in research, health, and nuclear industries.
Isotopic Water Purification
Raw water contains natural isotopic ratios of hydrogen and oxygen, including protium and deuterium, while deuterium depleted water (DDW) undergoes isotopic water purification processes that reduce the deuterium concentration below natural levels, typically below 130 ppm. Techniques such as fractional distillation, electrolysis, and catalytic exchange are employed to selectively separate heavy isotopes, enhancing the water's potential benefits in medical, agricultural, and industrial applications.
Light Water Filtration
Light water filtration targets the removal of impurities, including heavy isotopes like deuterium, to produce deuterium depleted water (DDW) with lower deuterium levels than raw water. This process enhances water quality by reducing hydrogen isotope concentration, benefiting metabolic and cellular functions where low deuterium content is advantageous.
D-D Water Ratio Index
Raw water typically contains a natural deuterium concentration of about 150 ppm, while Deuterium Depleted Water (DDW) has a significantly lower deuterium content, often below 120 ppm, enhancing its D-D Water Ratio Index by reducing isotopic mass differences. This optimized D-D Water Ratio Index in DDW supports improved biological and chemical processes by minimizing kinetic isotope effects commonly observed in raw water.
Quantum Hydration Effects
Raw water contains a natural isotope mix of hydrogen, including protium and deuterium, affecting molecular vibration and hydrogen bond dynamics. Deuterium depleted water (DDW) exhibits altered quantum hydration effects by lowering deuterium levels, enhancing proton tunneling rates and influencing water's structural and biochemical interactions at the quantum level.
Heavy Isotope Reduction
Raw water contains natural levels of heavy isotopes like deuterium, while deuterium depleted water (DDW) significantly reduces these heavy isotopes, lowering deuterium concentration below standard levels. This heavy isotope reduction in DDW enhances metabolic processes and improves cellular hydration compared to untreated raw water.
Raw Water vs Deuterium Depleted Water Infographic
 industrydif.com
industrydif.com