Vaccination remains the most effective method for preventing infectious diseases in pets by stimulating their immune system to recognize and fight pathogens before they cause illness. mRNA therapy, while promising in human medicine for conditions like cancer, is still experimental in veterinary care and not widely available for pets. Choosing vaccination offers established protection and a proven safety profile for maintaining pet health.
Table of Comparison
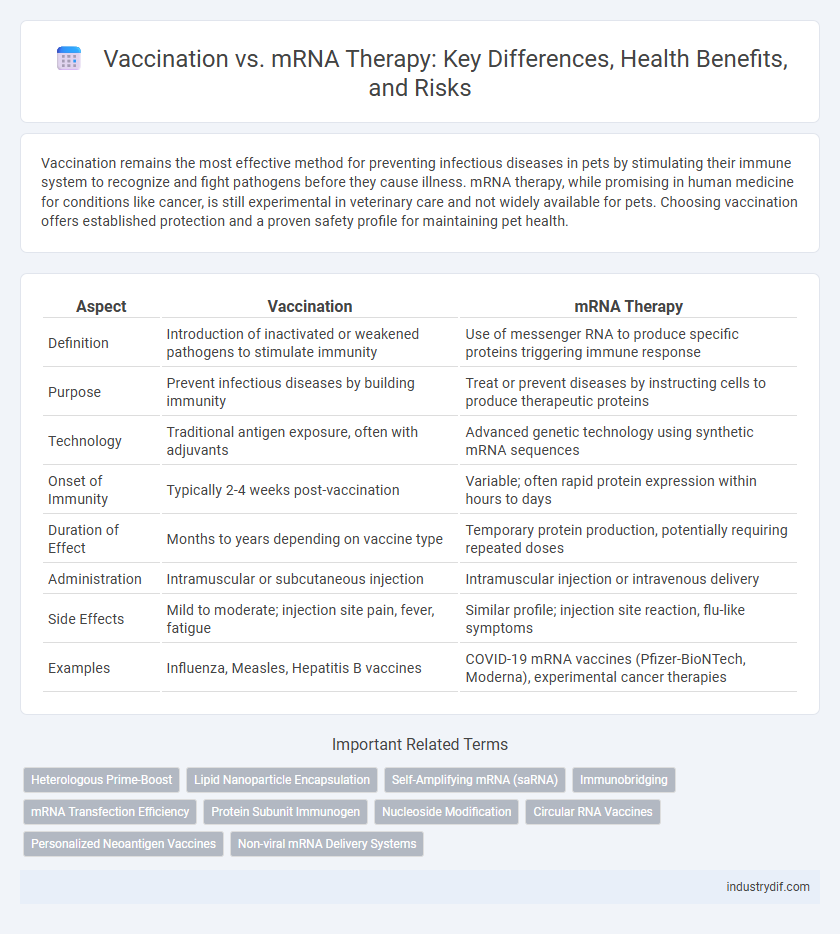
| Aspect | Vaccination | mRNA Therapy |
|---|---|---|
| Definition | Introduction of inactivated or weakened pathogens to stimulate immunity | Use of messenger RNA to produce specific proteins triggering immune response |
| Purpose | Prevent infectious diseases by building immunity | Treat or prevent diseases by instructing cells to produce therapeutic proteins |
| Technology | Traditional antigen exposure, often with adjuvants | Advanced genetic technology using synthetic mRNA sequences |
| Onset of Immunity | Typically 2-4 weeks post-vaccination | Variable; often rapid protein expression within hours to days |
| Duration of Effect | Months to years depending on vaccine type | Temporary protein production, potentially requiring repeated doses |
| Administration | Intramuscular or subcutaneous injection | Intramuscular injection or intravenous delivery |
| Side Effects | Mild to moderate; injection site pain, fever, fatigue | Similar profile; injection site reaction, flu-like symptoms |
| Examples | Influenza, Measles, Hepatitis B vaccines | COVID-19 mRNA vaccines (Pfizer-BioNTech, Moderna), experimental cancer therapies |
Overview of Traditional Vaccination
Traditional vaccination stimulates the immune system by introducing weakened or inactivated pathogens to trigger antibody production and long-term immunity. Common vaccines include those for measles, polio, and influenza, relying on established techniques such as live-attenuated and killed-virus formulations. These vaccines provide widespread protection by training the immune system to recognize and combat specific infectious agents effectively.
Understanding mRNA Therapy
mRNA therapy utilizes messenger RNA to instruct cells to produce specific proteins, enhancing the body's immune response without introducing live pathogens. Unlike traditional vaccines that often use weakened or inactive viruses, mRNA therapies offer targeted treatment for diseases by encoding protein production directly within cells. This innovative approach accelerates vaccine development and shows promise in treating various conditions, including cancer and genetic disorders.
Mechanisms of Action: Vaccines vs mRNA Therapies
Vaccination introduces weakened or inactive pathogens, stimulating the immune system to produce antibodies and memory cells against specific diseases. mRNA therapy delivers synthetic messenger RNA into cells, instructing them to produce targeted proteins that trigger an immune response or replace defective proteins. While vaccines primarily prevent infections by preparing the immune system, mRNA therapies enable precise protein synthesis for both therapeutic and preventive applications.
Development and Approval Processes
Vaccination development involves rigorous preclinical studies, phased clinical trials, and regulatory review to ensure safety and efficacy before public use. mRNA therapy undergoes a similar process but emphasizes platform adaptability and rapid modification capabilities to address emerging variants. Both methods require compliance with stringent guidelines from agencies like the FDA and EMA for approval and market authorization.
Efficacy in Disease Prevention
Vaccination delivers antigens that stimulate the immune system to produce a long-lasting protective response, effectively preventing infectious diseases such as measles and influenza. mRNA therapy instructs cells to produce specific proteins that can trigger an immune response or therapeutic effect, showing high efficacy in personalized cancer treatments and emerging viral infections. Comparative studies reveal that vaccines provide robust population-wide immunity, while mRNA therapies offer targeted and adaptable prevention strategies with rapid development timelines.
Safety Profiles and Side Effects
Vaccination and mRNA therapy both stimulate immune responses but differ in delivery and side effect profiles; vaccines often contain adjuvants and inactivated pathogens, while mRNA therapies use lipid nanoparticles to deliver genetic material. Common vaccine side effects include mild fever, fatigue, and localized pain, whereas mRNA therapy can cause transient inflammation and rare allergic reactions linked to lipid components. Safety profiles indicate both are generally well-tolerated, with ongoing monitoring critical to identify rare adverse events and ensure public health efficacy.
Storage and Distribution Challenges
Vaccination storage typically requires stringent cold chain conditions, with mRNA vaccines often demanding ultra-low temperatures between -80degC and -60degC to maintain stability and efficacy, complicating distribution logistics. mRNA therapies also face challenges in maintaining consistent temperature controls during transport, necessitating advanced refrigeration infrastructure and rapid delivery to healthcare facilities. These factors elevate costs and limit accessibility, particularly in low-resource and remote areas, impacting global immunization efforts and treatment availability.
Public Perception and Acceptance
Public perception of vaccination is generally more favorable due to long-standing evidence supporting its efficacy and safety, leading to higher acceptance rates. In contrast, mRNA therapy, despite its promising potential and rapid development, faces skepticism rooted in its novel technology and concerns about long-term effects. Effective communication emphasizing scientific validation and transparent data is critical to improving public trust and acceptance of mRNA-based treatments.
Regulatory and Ethical Considerations
Regulatory frameworks for vaccination emphasize rigorous clinical trials and long-term safety monitoring, ensuring widespread public trust and compliance. mRNA therapies face evolving guidelines due to their novel mechanisms, necessitating updated ethical standards to address informed consent and equitable access. Both approaches require ongoing oversight to balance innovation with patient safety and address disparities in healthcare delivery.
Future Directions in Immunization Technologies
mRNA therapy represents the cutting-edge frontier in immunization technologies, offering rapid adaptability against emerging pathogens compared to traditional vaccination platforms. Advances in delivery systems and personalized mRNA constructs are expected to enhance immune response specificity and minimize adverse effects. Integration of mRNA technology with AI-driven epitope prediction models promises to accelerate vaccine development and broaden protective coverage in future public health strategies.
Related Important Terms
Heterologous Prime-Boost
Heterologous prime-boost vaccination combines different vaccine platforms, such as traditional vaccines followed by mRNA therapy, enhancing immune response diversity and durability. Studies demonstrate that this approach can generate stronger neutralizing antibodies and T-cell responses compared to homologous regimens, improving protection against variants.
Lipid Nanoparticle Encapsulation
Lipid nanoparticle encapsulation enhances the delivery and stability of mRNA therapies by protecting the fragile mRNA molecules from degradation and facilitating efficient cellular uptake. This technology also plays a crucial role in mRNA vaccines by ensuring targeted delivery of antigen-encoding mRNA to immune cells, thereby triggering robust immune responses.
Self-Amplifying mRNA (saRNA)
Self-amplifying mRNA (saRNA) therapy enhances traditional mRNA vaccines by replicating intracellularly, enabling lower dosages and prolonged protein expression for improved immune responses. This innovative approach offers significant potential in infectious disease prevention and personalized medicine by stimulating robust and durable immunity.
Immunobridging
Immunobridging evaluates immune responses generated by traditional vaccination compared to mRNA therapy, enabling assessment of vaccine efficacy without large-scale efficacy trials. This method uses antibody titers and T-cell responses as surrogate markers to expedite approval of new vaccine formulations and variants.
mRNA Transfection Efficiency
mRNA transfection efficiency plays a critical role in mRNA therapy effectiveness, directly impacting protein expression levels and therapeutic outcomes compared to traditional vaccination methods. Optimizing lipid nanoparticle formulations and delivery techniques enhances cellular uptake and endosomal escape, thereby improving the potency and reliability of mRNA-based treatments.
Protein Subunit Immunogen
Protein subunit immunogens used in vaccination deliver purified antigenic proteins to stimulate a targeted immune response without introducing genetic material, unlike mRNA therapy which instructs cells to produce antigen proteins internally. This direct antigen presentation in protein subunit vaccines enhances safety by reducing the risk of unintended cellular processes associated with mRNA platforms while maintaining effective immunogenicity.
Nucleoside Modification
Nucleoside modification in mRNA therapy enhances stability and reduces immunogenicity, enabling efficient protein translation without triggering excessive immune responses. In contrast, traditional vaccination relies on introducing weakened pathogens or antigens to stimulate immunity, lacking the precision and rapid adaptability offered by nucleoside-modified mRNA platforms.
Circular RNA Vaccines
Circular RNA vaccines offer enhanced stability and prolonged antigen expression compared to traditional mRNA therapies, potentially improving immune response durability. Their unique closed-loop structure resists degradation enzymes, enabling efficient protein translation critical for effective vaccination against infectious diseases.
Personalized Neoantigen Vaccines
Personalized neoantigen vaccines harness tumor-specific mutations to elicit a targeted immune response, enhancing the efficacy of cancer immunotherapy compared to traditional mRNA therapies that broadly stimulate immune activation. This precision approach leverages next-generation sequencing and bioinformatics to design individualized vaccines that optimize T-cell recognition and minimize off-target effects, advancing personalized medicine in oncology.
Non-viral mRNA Delivery Systems
Non-viral mRNA delivery systems, such as lipid nanoparticles (LNPs) and polymer-based carriers, enable effective vaccination by ensuring targeted cellular uptake and minimizing immune reactions, unlike traditional viral vectors. These platforms enhance mRNA stability, facilitate controlled release, and offer scalable, safe alternatives in both prophylactic vaccines and mRNA-based therapeutic interventions.
Vaccination vs mRNA Therapy Infographic
 industrydif.com
industrydif.com