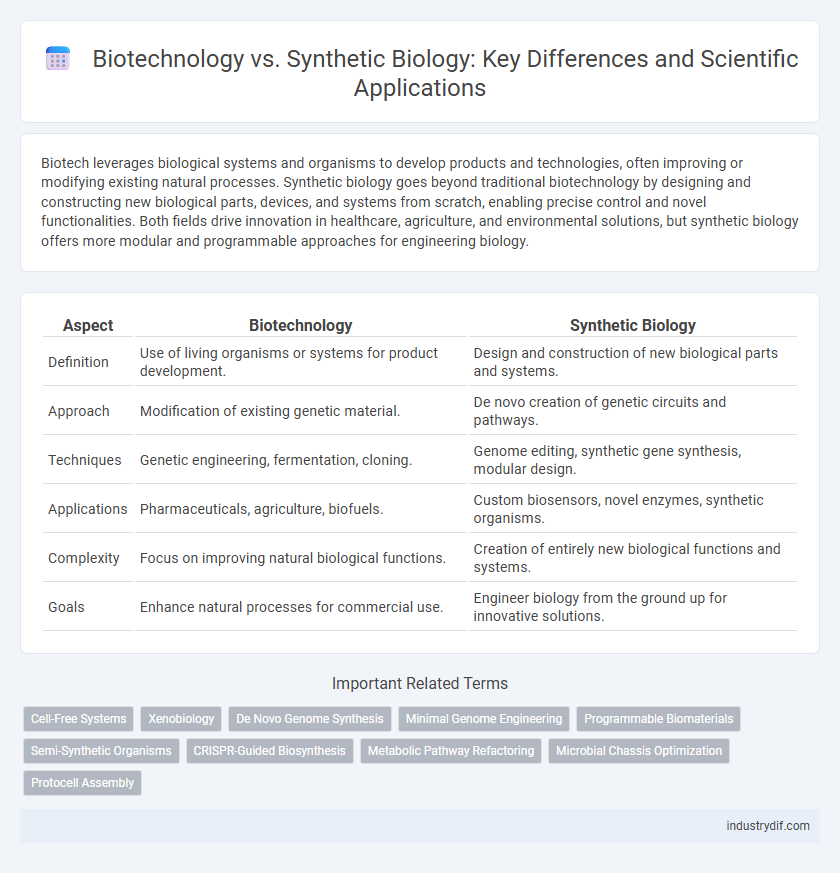
Biotech leverages biological systems and organisms to develop products and technologies, often improving or modifying existing natural processes. Synthetic biology goes beyond traditional biotechnology by designing and constructing new biological parts, devices, and systems from scratch, enabling precise control and novel functionalities. Both fields drive innovation in healthcare, agriculture, and environmental solutions, but synthetic biology offers more modular and programmable approaches for engineering biology.
Table of Comparison
| Aspect | Biotechnology | Synthetic Biology |
|---|---|---|
| Definition | Use of living organisms or systems for product development. | Design and construction of new biological parts and systems. |
| Approach | Modification of existing genetic material. | De novo creation of genetic circuits and pathways. |
| Techniques | Genetic engineering, fermentation, cloning. | Genome editing, synthetic gene synthesis, modular design. |
| Applications | Pharmaceuticals, agriculture, biofuels. | Custom biosensors, novel enzymes, synthetic organisms. |
| Complexity | Focus on improving natural biological functions. | Creation of entirely new biological functions and systems. |
| Goals | Enhance natural processes for commercial use. | Engineer biology from the ground up for innovative solutions. |
Defining Biotech and Synthetic Biology
Biotechnology involves using living organisms or biological systems to develop products and technologies that improve health, agriculture, and industry. Synthetic biology is an advanced subset of biotechnology that combines engineering principles with biology to design and construct new biological parts, devices, and systems not found in nature. This field leverages genetic engineering, computer modeling, and synthetic DNA to create novel organisms and functions for specific applications.
Historical Development of Biotech and Synthetic Biology
Biotechnology emerged in the early 20th century with advances in microbiology and genetic engineering, exemplified by the development of recombinant DNA technology in the 1970s. Synthetic biology evolved later, around the early 2000s, integrating engineering principles with biology to design and construct new biological parts, devices, and systems. Key milestones include the synthesis of the first artificial genome by the J. Craig Venter Institute in 2010, marking a significant advancement beyond traditional biotech techniques.
Core Technologies in Biotech and Synthetic Biology
Core technologies in biotechnology revolve around genetic engineering, recombinant DNA technology, and fermentation processes that enable precise manipulation of biological systems for pharmaceuticals and agriculture. Synthetic biology extends these foundations by integrating computational modeling, standardized genetic parts, and genome editing tools such as CRISPR-Cas systems to design and construct new biological functions and organisms. While biotechnology emphasizes modifying existing biological systems, synthetic biology focuses on creating novel biological components and systems through an engineering approach.
Key Differences in Methodology
Biotechnology primarily utilizes living organisms and biological processes to develop products or technologies, often relying on existing natural pathways and genetic material. Synthetic biology, in contrast, employs engineering principles to design and construct novel biological systems or organisms from scratch, using standardized genetic parts and modular components. While biotechnology modifies organisms for practical applications, synthetic biology innovates by creating entirely new biological functions through precise genetic circuit design.
Applications in Healthcare and Medicine
Biotechnology encompasses the use of living organisms and biological systems for medical advancements, including gene therapy, vaccine development, and diagnostic tools. Synthetic biology enhances these applications by designing and constructing new biological parts, such as synthetic genes and engineered cells, enabling personalized medicine and advanced drug delivery systems. Integrating synthetic biology with traditional biotech accelerates innovations in regenerative medicine and targeted cancer therapies.
Industrial and Environmental Applications
Biotechnology harnesses living organisms and biological processes for industrial applications such as biofuel production, pharmaceuticals, and waste treatment, driving sustainable solutions. Synthetic biology advances these capabilities by designing and constructing new biological parts and systems, enabling precision manufacturing of chemicals, biodegradable materials, and enhanced environmental remediation. Both fields contribute to reducing industrial pollution and promoting eco-friendly technologies through innovative biological tools and engineered organisms.
Regulatory and Ethical Considerations
Biotechnology regulations primarily address genetic modification, biosafety, and patenting while emphasizing risk assessment and environmental impact to ensure safe application. Synthetic biology presents unique ethical challenges involving dual-use research, biosecurity risks, and synthetic life creation, necessitating updated frameworks to manage potential societal consequences. Regulatory bodies must harmonize guidelines with scientific advancements to balance innovation, public health, and ethical integrity in both fields.
Investment and Market Trends
Investment in biotech has surged to over $50 billion globally in 2023, driven by advancements in gene editing and personalized medicine. Synthetic biology, a rapidly growing subset, attracted $8 billion in venture capital, emphasizing sustainable solutions and bio-manufacturing innovations. Market trends reveal synthetic biology's higher growth rate of 25% annually, outpacing the broader biotech sector's 12% expansion.
Future Prospects and Innovations
Biotech continues to advance by integrating gene editing, personalized medicine, and bioinformatics to drive sustainable solutions in healthcare and agriculture. Synthetic biology pushes these boundaries further by designing and constructing novel biological parts, systems, and organisms, enabling breakthroughs in biomanufacturing and environmental remediation. Future innovations will likely emerge from the convergence of both fields, accelerating the development of precision therapies, synthetic genomes, and scalable biofactories.
Challenges and Limitations
Biotech faces challenges such as limited genetic toolkits and ethical concerns related to genetic modification, constraining its applications in medicine and agriculture. Synthetic biology encounters limitations in standardizing biological parts and managing complex system unpredictability, which hampers scalability and commercial deployment. Both fields must address biosafety risks and regulatory hurdles to advance innovation and public acceptance.
Related Important Terms
Cell-Free Systems
Cell-free systems enable synthetic biology by providing a controlled environment for protein synthesis without living cells, enhancing scalability and precision in biotechnological applications. These systems bridge traditional biotech and synthetic biology by accelerating pathway engineering, reducing complexity, and improving reproducibility in biomolecular production.
Xenobiology
Xenobiology, a subfield of synthetic biology, engineering novel biological systems using non-natural nucleotides and amino acids to create organisms with expanded genetic codes, contrasts with traditional biotechnology that primarily modifies existing biological systems. This innovative approach offers potential breakthroughs in drug development, biosafety, and biocontainment by producing life forms with orthogonal biochemical pathways not found in nature.
De Novo Genome Synthesis
De novo genome synthesis in synthetic biology enables the construction of entirely new genetic sequences from scratch, offering precise control over genome design compared to traditional biotech methods that primarily modify existing organisms. This approach accelerates innovation in creating custom microorganisms with tailored functions for applications in medicine, agriculture, and environmental sustainability.
Minimal Genome Engineering
Minimal genome engineering in synthetic biology involves precisely editing or reducing an organism's genome to essential genes for desired functions, enabling streamlined chassis development. In contrast, traditional biotechnology often utilizes whole organisms or cells without extensive genomic reduction, limiting optimization and efficiency in engineered biological systems.
Programmable Biomaterials
Programmable biomaterials, central to synthetic biology, enable precise control over molecular architecture and function by integrating engineered biological components, surpassing traditional biotech approaches that primarily rely on natural biological systems. These advanced materials facilitate innovations in tissue engineering, drug delivery, and biosensing through customizable and responsive properties tailored at the genetic and protein levels.
Semi-Synthetic Organisms
Semi-synthetic organisms represent a convergence of biotechnology and synthetic biology, integrating synthetic genetic components into living cells to expand their natural capabilities. These engineered organisms leverage modified DNA bases or non-standard nucleotides to enhance biological functions, enabling advances in drug development, biomanufacturing, and environmental sensing.
CRISPR-Guided Biosynthesis
CRISPR-guided biosynthesis enhances synthetic biology by enabling precise genome editing to design and construct novel biological pathways, surpassing traditional biotech methods that rely on naturally occurring processes. This technology facilitates targeted manipulation of genetic material, accelerating the development of custom enzymes, metabolites, and therapeutic compounds with unprecedented accuracy and efficiency.
Metabolic Pathway Refactoring
Metabolic pathway refactoring in synthetic biology involves redesigning and optimizing genetic circuits to enhance microbial production of valuable compounds, surpassing traditional biotech methods that primarily focus on natural pathway engineering. This approach leverages advanced gene editing tools and computational modeling to reconstruct pathways with improved efficiency, yield, and robustness in host organisms.
Microbial Chassis Optimization
Microbial chassis optimization in synthetic biology involves precise genome editing and pathway engineering to enhance metabolic efficiency and product yield, surpassing traditional biotech methods reliant on less customizable microbial strains. Advanced tools like CRISPR-Cas systems enable synthetic biology to tailor microbial hosts for specific bioproduction tasks, driving innovation in pharmaceuticals, biofuels, and industrial enzymes.
Protocell Assembly
Protocell assembly in synthetic biology emphasizes designing minimal, functional cell-like systems using synthetic components, whereas biotechnology often utilizes existing biological frameworks for engineering purposes. Advances in protocell construction enable precise control over molecular self-assembly, membrane formation, and encapsulation, enhancing the development of artificial cells with tailored functions.
Biotech vs Synthetic Biology Infographic
 industrydif.com
industrydif.com