Model organisms provide invaluable insights into biological processes through whole-animal studies, enabling researchers to observe systemic interactions and genetic functions in vivo. Organ-on-a-chip technology offers a microengineered platform that replicates organ-specific microenvironments, allowing precise control over cellular behaviors and real-time monitoring of physiological responses. Combining both approaches enhances drug development and disease modeling by bridging organism-level complexity with organ-specific mechanistic understanding.
Table of Comparison
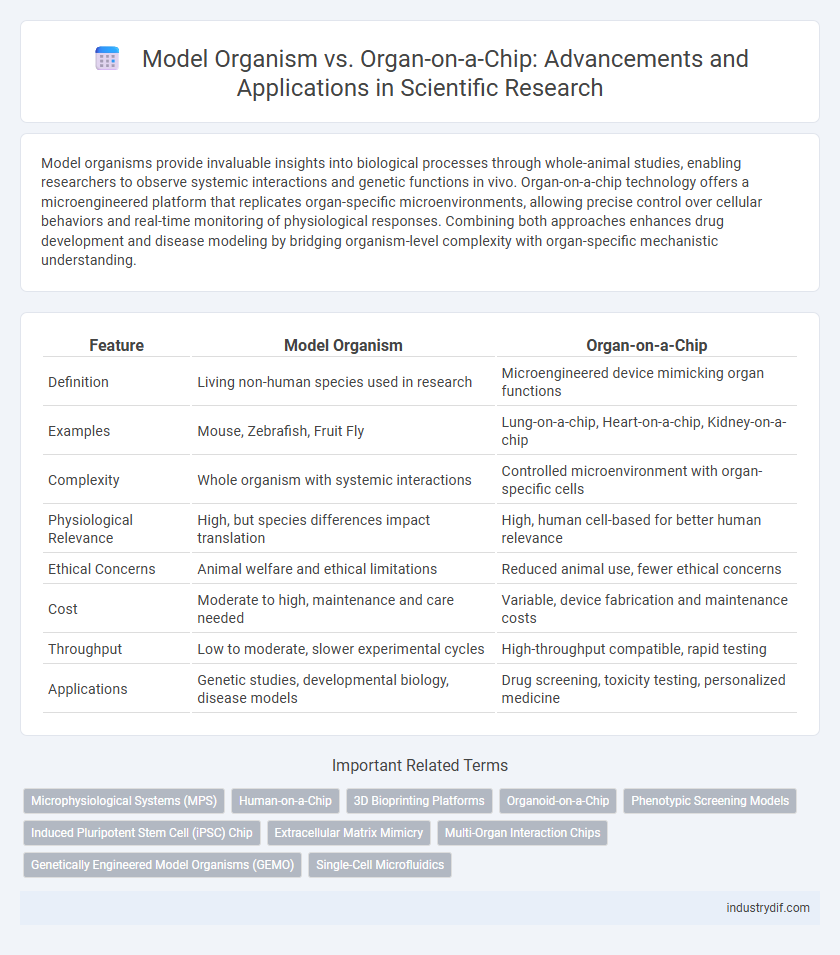
| Feature | Model Organism | Organ-on-a-Chip |
|---|---|---|
| Definition | Living non-human species used in research | Microengineered device mimicking organ functions |
| Examples | Mouse, Zebrafish, Fruit Fly | Lung-on-a-chip, Heart-on-a-chip, Kidney-on-a-chip |
| Complexity | Whole organism with systemic interactions | Controlled microenvironment with organ-specific cells |
| Physiological Relevance | High, but species differences impact translation | High, human cell-based for better human relevance |
| Ethical Concerns | Animal welfare and ethical limitations | Reduced animal use, fewer ethical concerns |
| Cost | Moderate to high, maintenance and care needed | Variable, device fabrication and maintenance costs |
| Throughput | Low to moderate, slower experimental cycles | High-throughput compatible, rapid testing |
| Applications | Genetic studies, developmental biology, disease models | Drug screening, toxicity testing, personalized medicine |
Introduction to Model Organisms and Organ-on-a-Chip
Model organisms such as fruit flies, mice, and zebrafish provide essential biological insights due to their genetic similarities to humans and well-characterized genomes. Organ-on-a-chip technology integrates microfluidics and human cell cultures to replicate organ-level functions, enabling more precise modeling of physiological responses. These advanced platforms complement traditional model organisms by offering enhanced control over cellular environments and real-time analysis of biochemical processes.
Historical Development and Scientific Milestones
Model organisms such as Drosophila melanogaster and Caenorhabditis elegans have been instrumental since the early 20th century for elucidating fundamental genetic and developmental processes, with milestones including the discovery of the DNA double helix structure and the development of gene-editing techniques like CRISPR. Organ-on-a-chip technology emerged in the early 21st century, integrating microfluidics and cellular biology to simulate organ-level physiology and pathophysiology, achieving significant breakthroughs such as recapitulating lung inflammation and cardiac tissue function in vitro. Both platforms have transformed biomedical research, with model organisms providing in vivo insights and organ-on-a-chip devices enabling precise control of microenvironments for drug testing and disease modeling.
Biological Relevance: Whole-Organism vs Microengineered Systems
Model organisms provide whole-organism biological relevance by encompassing complex systemic interactions and physiological responses inherent to living beings. Organ-on-a-chip systems offer microengineered, biomimetic environments that replicate specific organ functions with high precision, enabling detailed cellular and molecular studies. Combining insights from whole-organism models and organ-on-a-chip technology enhances the understanding of biological processes while reducing reliance on animal models.
Comparative Advantages in Biomedical Research
Model organisms offer well-established genetic and physiological data supporting broad biological insights, while organ-on-a-chip devices provide precise microenvironmental control and human-relevant tissue modeling for more accurate disease simulation and drug testing. Organ-on-a-chip technology reduces interspecies variations and enhances predictive validity for human responses, addressing limitations inherent in animal models. The integration of both approaches optimizes translational research by combining systemic complexity with high-throughput, mechanistic analysis.
Limitations and Ethical Considerations
Model organisms often face limitations such as species-specific differences that hinder translational accuracy to human biology, alongside ethical concerns related to animal welfare. Organ-on-a-chip technology presents a more ethical alternative by reducing animal use and better mimicking human physiological responses, yet it struggles with complex systemic interactions and scalability issues. Both models require careful consideration of ethical implications and scientific limitations to optimize their use in biomedical research.
Applications in Disease Modeling and Drug Discovery
Model organisms such as mice and zebrafish provide in vivo systems essential for studying complex disease mechanisms and systemic drug effects, allowing researchers to observe physiological interactions in a whole organism context. Organ-on-a-chip technology offers precise microfluidic platforms that mimic human organ-level functions, enabling more accurate modeling of disease pathology and patient-specific drug responses at the cellular and tissue scales. Combining these approaches enhances drug discovery pipelines by integrating whole-organism biological complexity with controlled microenvironment simulations, improving predictive power and translational relevance in biomedical research.
Scalability and Throughput in Experimental Design
Model organisms offer established scalability for high-throughput screening due to their well-characterized genetics and ease of replication in large cohorts. Organ-on-a-chip technology enables scalable experimentation with microfluidic platforms that simulate human organ functions, providing higher physiological relevance at potentially lower sample volumes. Throughput in organ-on-a-chip systems can be enhanced by parallelization of chips, while model organisms benefit from rapid breeding cycles to generate large sample sizes.
Integration with Emerging Technologies
Model organisms, such as mice and zebrafish, have been foundational in biological research due to their genetic similarity to humans and ease of manipulation, yet their scalability faces limits in mimicking complex human physiology. Organ-on-a-chip technology integrates microfluidics, tissue engineering, and sensor systems to create biomimetic environments that replicate human organ functions at microscale, enabling precise control over cellular microenvironments and real-time monitoring. Emerging technologies like machine learning and high-throughput screening enhance organ-on-a-chip platforms by optimizing experimental parameters and accelerating drug discovery processes beyond the constraints of traditional model organisms.
Regulatory Perspectives and Translational Potential
Regulatory agencies increasingly recognize Organ-on-a-Chip technology for its ability to replicate human physiological responses more accurately than traditional model organisms, enhancing translational predictability. While model organisms like mice provide essential in vivo insights, their interspecies differences limit direct application to human biology in drug development and toxicity testing. Organ-on-a-Chip platforms offer scalable, human-relevant data that align with regulatory frameworks aiming to reduce animal testing and improve clinical trial success rates.
Future Trends in Preclinical Research Platforms
Emerging preclinical research platforms increasingly favor organ-on-a-chip technology due to its ability to replicate human physiology and disease microenvironments with higher precision than traditional model organisms such as mice and zebrafish. Advances in microfluidics, 3D cell culture, and biomaterials enhance organ-on-a-chip models, enabling real-time monitoring of cellular responses and drug interactions under dynamic flow conditions that better mimic in vivo states. The integration of artificial intelligence and high-throughput screening further accelerates personalized medicine development and reduces reliance on animal models, driving a paradigm shift toward more predictive and ethical drug discovery methodologies.
Related Important Terms
Microphysiological Systems (MPS)
Microphysiological Systems (MPS) integrate Organ-on-a-Chip technology to replicate human tissue interfaces and physiological responses more accurately than traditional model organisms, enhancing predictive validity for drug development. These systems leverage microfluidic channels and human cells to mimic organ-level functions, reducing reliance on animal models and improving translational research outcomes.
Human-on-a-Chip
Human-on-a-Chip systems integrate multiple organ-on-a-chip devices to simulate human physiological responses with higher accuracy than traditional model organisms, enabling precise studies of drug metabolism, toxicity, and disease mechanisms. These microfluidic platforms replicate inter-organ interactions and dynamic biological environments, offering improved translational relevance for biomedical research and personalized medicine.
3D Bioprinting Platforms
3D bioprinting platforms enable the precise fabrication of organ-on-a-chip models that mimic human tissue architecture and physiological functions more accurately than traditional model organisms. These advanced systems facilitate high-throughput drug screening and disease modeling by integrating multiple cell types within microfluidic environments, overcoming species-specific limitations inherent to animal models.
Organoid-on-a-Chip
Organoid-on-a-chip integrates 3D organoid cultures with microfluidic technology, enhancing physiological relevance and allowing dynamic control of the cellular microenvironment compared to traditional model organisms. This advanced platform enables precise studies of organ-level functions, disease modeling, and drug responses, overcoming limitations of static organoids and animal models.
Phenotypic Screening Models
Phenotypic screening models using model organisms like zebrafish and C. elegans provide whole-organism insights into drug effects, offering complex systemic interactions and genetic relevance. Organ-on-a-Chip platforms enable high-throughput, microfluidic-based phenotypic assays with human cell specificity, capturing tissue-level responses and improving translational accuracy in preclinical screening.
Induced Pluripotent Stem Cell (iPSC) Chip
Induced pluripotent stem cell (iPSC) chips offer a cutting-edge platform that replicates human tissue complexity more accurately than traditional model organisms, enhancing disease modeling and drug testing precision. These Organ-on-a-Chip systems integrate patient-specific iPSCs to simulate physiological responses, enabling personalized medicine approaches and reducing reliance on animal models.
Extracellular Matrix Mimicry
Model organisms offer valuable insights into biological processes but often lack precise extracellular matrix (ECM) mimicry, limiting their relevance in replicating human tissue environments. Organ-on-a-chip technologies incorporate engineered ECM components, providing microscale architectures that better simulate cellular interactions and biomechanical cues found in native human tissues.
Multi-Organ Interaction Chips
Multi-organ interaction chips replicate complex physiological connectivity by integrating several organ-specific cell cultures on microfluidic platforms, enhancing the predictive accuracy of human responses compared to traditional model organisms like mice or zebrafish. These Organ-on-a-Chip systems enable precise control over biochemical gradients and mechanical stimuli, facilitating advanced studies of inter-organ communication, drug metabolism, and systemic toxicity in a human-relevant setting.
Genetically Engineered Model Organisms (GEMO)
Genetically Engineered Model Organisms (GEMOs) provide precise genetic manipulation to study disease pathways and gene function, enhancing translational relevance in biomedical research. Organ-on-a-chip technologies complement GEMOs by mimicking human physiological responses at the microfluidic level, enabling dynamic interaction studies that traditional GEMOs cannot replicate.
Single-Cell Microfluidics
Single-cell microfluidics enhances the resolution of cellular analysis in model organisms by enabling precise manipulation and monitoring of individual cells, whereas organ-on-a-chip systems replicate microphysiological environments to mimic organ-level functions through integrated microfluidic channels. These technologies provide complementary advantages for high-throughput screening and disease modeling, with single-cell microfluidics offering granular insights into cellular heterogeneity and organ-on-a-chip platforms facilitating dynamic tissue-like interactions.
Model Organism vs Organ-on-a-Chip Infographic
 industrydif.com
industrydif.com