Clinical trials remain the gold standard for validating new treatments by testing on real patients to collect empirical data, while digital twins create precise virtual models of individual patients to simulate responses and optimize therapies. Digital twins enhance trial efficiency by enabling personalized predictions and reducing risks, offering a scalable complement to traditional methods. Integrating digital twins with clinical trials accelerates drug development, improves outcome accuracy, and supports data-driven decision-making in scientific pet care.
Table of Comparison
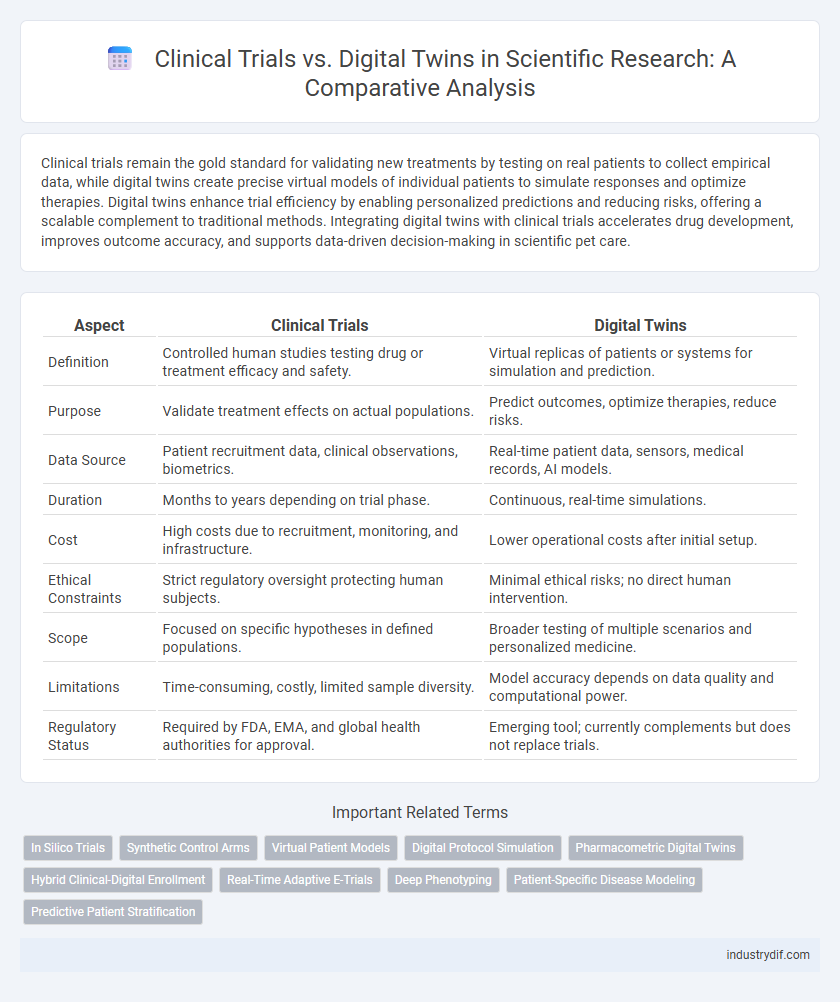
| Aspect | Clinical Trials | Digital Twins |
|---|---|---|
| Definition | Controlled human studies testing drug or treatment efficacy and safety. | Virtual replicas of patients or systems for simulation and prediction. |
| Purpose | Validate treatment effects on actual populations. | Predict outcomes, optimize therapies, reduce risks. |
| Data Source | Patient recruitment data, clinical observations, biometrics. | Real-time patient data, sensors, medical records, AI models. |
| Duration | Months to years depending on trial phase. | Continuous, real-time simulations. |
| Cost | High costs due to recruitment, monitoring, and infrastructure. | Lower operational costs after initial setup. |
| Ethical Constraints | Strict regulatory oversight protecting human subjects. | Minimal ethical risks; no direct human intervention. |
| Scope | Focused on specific hypotheses in defined populations. | Broader testing of multiple scenarios and personalized medicine. |
| Limitations | Time-consuming, costly, limited sample diversity. | Model accuracy depends on data quality and computational power. |
| Regulatory Status | Required by FDA, EMA, and global health authorities for approval. | Emerging tool; currently complements but does not replace trials. |
Introduction to Clinical Trials and Digital Twins
Clinical trials are systematic investigations involving human participants designed to evaluate the safety and efficacy of new medical treatments, drugs, or interventions under controlled conditions. Digital twins in healthcare refer to sophisticated virtual replicas of patients or biological systems created using real-time data and advanced simulations to predict health outcomes and optimize personalized treatments. Incorporating digital twins into clinical trials can enhance study design, patient stratification, and predictive analytics, ultimately accelerating drug development and reducing costs.
Evolution of Research: From Clinical Trials to Digital Twins
Clinical trials have historically provided critical data on drug efficacy and safety through controlled, population-based studies, but their limitations include high costs, lengthy timelines, and variability in patient responses. Digital twins, virtual replicas of patients generated from multi-dimensional health data, are transforming research by enabling real-time simulation of treatment effects and personalized medicine predictions. This evolution enhances precision in clinical decision-making, accelerates drug development, and reduces reliance on traditional trial-and-error methodologies.
Defining Clinical Trials: Methodologies and Applications
Clinical trials employ randomized controlled methodologies to assess the safety and efficacy of new medical treatments, ensuring robust data through participant monitoring and statistical analysis. These trials are essential for regulatory approval, involving phases from initial safety testing to large-scale efficacy evaluation. Applications span drug development, medical devices, and behavioral interventions, providing empirical evidence for clinical decision-making and healthcare innovation.
What Are Digital Twins in Healthcare?
Digital twins in healthcare are virtual replicas of physical patients or biological systems that simulate real-time physiological processes using data from wearable devices, electronic health records, and imaging technologies. These models enable personalized treatment planning, predictive diagnostics, and virtual testing, reducing the need for traditional clinical trial methods. By integrating AI and machine learning, digital twins enhance precision medicine by continuously updating patient-specific simulations based on new health data.
Key Differences: Clinical Trials vs Digital Twins
Clinical trials involve testing new treatments on human participants to evaluate safety and efficacy, while digital twins utilize virtual replicas of biological systems to simulate and predict outcomes without physical testing. Clinical trials provide real-world, empirical data but are time-consuming and expensive, whereas digital twins enable rapid hypothesis testing and optimization through computational models. The integration of digital twins can enhance clinical trial design by reducing risk and refining patient stratification strategies.
Benefits of Digital Twins Over Traditional Clinical Trials
Digital twins offer enhanced precision by simulating patient-specific conditions and treatment responses, reducing the variability often encountered in traditional clinical trials. They enable real-time data integration from wearable devices and sensors, accelerating the evaluation of drug efficacy and safety while minimizing the need for large patient cohorts. By facilitating virtual testing environments, digital twins significantly lower costs and ethical concerns associated with human trials, expediting the drug development process.
Challenges and Limitations of Digital Twins in Research
Digital twins in research face significant challenges, including high computational demands and the need for extensive, high-quality data to ensure accurate simulations. Limitations also arise from the difficulty in replicating complex biological systems and patient variability, which can reduce predictive reliability. Unlike traditional clinical trials, digital twins lack standardized validation protocols, creating hurdles for regulatory acceptance and widespread adoption.
Integrating Digital Twins into Clinical Trial Design
Integrating digital twins into clinical trial design enhances patient-specific modeling by simulating individual physiological responses, thereby reducing trial durations and improving outcome predictability. This approach leverages real-time data aggregation and advanced computational algorithms to optimize dose selection and minimize adverse effects. Digital twins enable adaptive trial protocols that increase statistical power and reduce the need for large patient cohorts, fundamentally transforming traditional clinical research methodologies.
Regulatory Considerations for Digital Twins and Clinical Trials
Regulatory considerations for digital twins in clinical trials involve ensuring data integrity, validation of digital models, and compliance with guidelines such as FDA's Digital Health Innovation Action Plan. Unlike traditional clinical trials governed by strict protocols and phases, digital twin technology requires continuous monitoring and adaptive regulatory frameworks to address real-time simulation accuracy and patient safety. Regulatory agencies emphasize transparency in algorithm development, secure data handling, and interoperability standards to validate digital twin outputs as reliable evidence in medical decision-making.
Future Perspectives: The Converging Path of Clinical Trials and Digital Twins
The convergence of clinical trials and digital twins promises to transform personalized medicine by enabling more accurate patient simulations and predictive modeling. Digital twins facilitate virtual trial protocols, reducing costs and accelerating drug development timelines while enhancing safety and efficacy assessments. Future perspectives emphasize integrating real-time patient data with computational models to optimize trial designs and improve treatment outcomes.
Related Important Terms
In Silico Trials
In silico trials leverage digital twins to simulate human physiology and disease progression, enabling rapid and cost-effective evaluation of medical treatments compared to traditional clinical trials. These computational models improve prediction accuracy for drug efficacy and patient-specific responses, reducing the need for extensive human subject testing.
Synthetic Control Arms
Synthetic control arms generated through digital twin technology provide a dynamic, patient-specific alternative to traditional clinical trial control groups by using real-world data and predictive modeling. This approach reduces reliance on placebo groups, accelerates trial timelines, and enhances the precision of efficacy and safety assessments in drug development.
Virtual Patient Models
Virtual patient models in digital twins enable dynamic simulation of individualized physiological responses, offering enhanced precision and adaptability compared to traditional clinical trials. These models reduce the need for extensive human testing by predicting treatment outcomes and optimizing trial designs through real-time data integration and advanced machine learning algorithms.
Digital Protocol Simulation
Digital protocol simulation using digital twins enables precise modeling of clinical trial scenarios, significantly reducing time and costs associated with patient recruitment and trial execution. This technology enhances predictive accuracy of treatment outcomes by replicating physiological responses in virtual patients, improving trial design and decision-making processes.
Pharmacometric Digital Twins
Pharmacometric digital twins use computational models to simulate individual patient responses, enabling personalized drug dosing and treatment optimization beyond traditional clinical trials. These digital replicas integrate real-time patient data and pharmacokinetic-pharmacodynamic models, accelerating hypothesis testing and reducing the need for extensive in vivo studies.
Hybrid Clinical-Digital Enrollment
Hybrid clinical-digital enrollment integrates real-time patient data from digital twins to optimize participant selection, enhancing trial accuracy and reducing recruitment timelines. This approach leverages predictive modeling from digital twins to simulate patient responses, improving trial efficiency and personalized treatment validation.
Real-Time Adaptive E-Trials
Real-time adaptive e-trials leverage digital twins to simulate patient responses dynamically, enabling more precise and efficient modifications to clinical trial protocols. This innovative approach enhances data accuracy, reduces trial duration, and improves personalized treatment outcomes compared to traditional clinical trials.
Deep Phenotyping
Deep phenotyping enhances clinical trials by providing comprehensive, precise patient data, enabling tailored treatment strategies and improved outcome prediction. Digital twins leverage this detailed phenotypic information to create dynamic virtual models of patients, facilitating personalized simulations and accelerating drug development processes.
Patient-Specific Disease Modeling
Clinical trials traditionally rely on large patient cohorts to assess treatment efficacy, whereas digital twins enable precise patient-specific disease modeling by simulating individual physiological responses using real-time data and advanced computational algorithms. This approach enhances personalized medicine by predicting disease progression and treatment outcomes at an individual level, reducing the time and cost associated with conventional clinical trials.
Predictive Patient Stratification
Predictive patient stratification in clinical trials leverages digital twins to simulate individual patient responses, enhancing precision in treatment personalization and reducing trial variability. By integrating real-time biometric data and advanced AI models, digital twins enable accurate prediction of clinical outcomes, accelerating patient selection and optimizing resource allocation.
Clinical Trials vs Digital Twins Infographic
 industrydif.com
industrydif.com