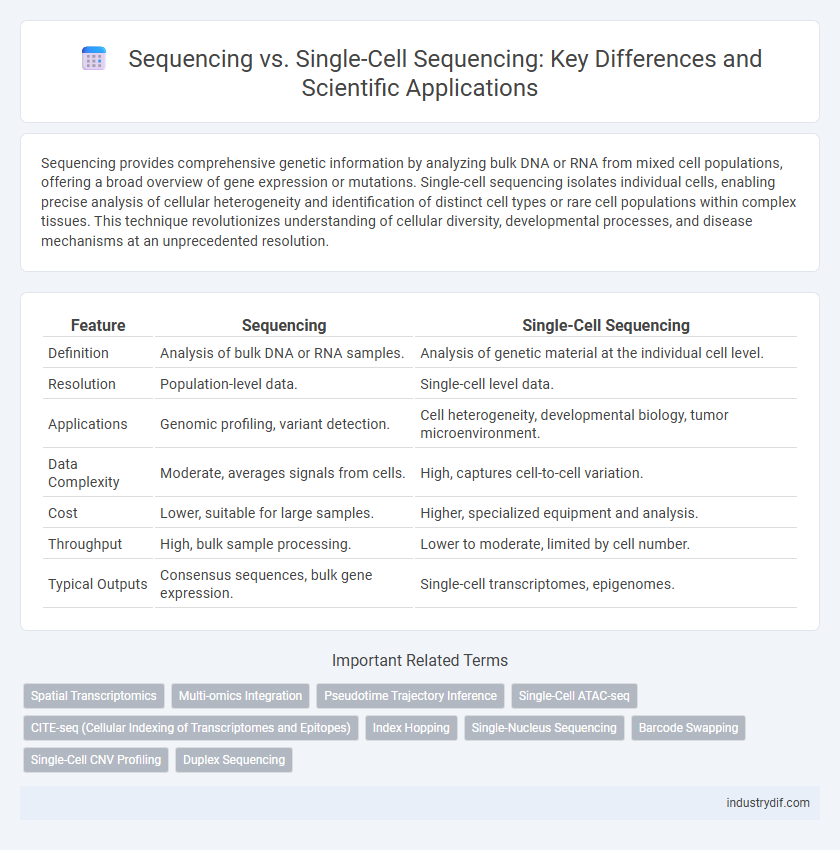
Sequencing provides comprehensive genetic information by analyzing bulk DNA or RNA from mixed cell populations, offering a broad overview of gene expression or mutations. Single-cell sequencing isolates individual cells, enabling precise analysis of cellular heterogeneity and identification of distinct cell types or rare cell populations within complex tissues. This technique revolutionizes understanding of cellular diversity, developmental processes, and disease mechanisms at an unprecedented resolution.
Table of Comparison
| Feature | Sequencing | Single-Cell Sequencing |
|---|---|---|
| Definition | Analysis of bulk DNA or RNA samples. | Analysis of genetic material at the individual cell level. |
| Resolution | Population-level data. | Single-cell level data. |
| Applications | Genomic profiling, variant detection. | Cell heterogeneity, developmental biology, tumor microenvironment. |
| Data Complexity | Moderate, averages signals from cells. | High, captures cell-to-cell variation. |
| Cost | Lower, suitable for large samples. | Higher, specialized equipment and analysis. |
| Throughput | High, bulk sample processing. | Lower to moderate, limited by cell number. |
| Typical Outputs | Consensus sequences, bulk gene expression. | Single-cell transcriptomes, epigenomes. |
Introduction to Sequencing Technologies
Sequencing technologies enable the decoding of nucleic acid sequences, providing insights into genetic information and molecular biology. Traditional sequencing methods, such as Sanger sequencing and next-generation sequencing (NGS), focus on bulk samples, capturing averaged genomic data from a cell population. Single-cell sequencing techniques, including single-cell RNA-seq and single-cell DNA-seq, allow high-resolution analysis at the individual cell level, revealing cellular heterogeneity and dynamic biological processes.
Fundamentals of Bulk Sequencing
Bulk sequencing analyzes DNA or RNA from a mixture of cells, providing an average genomic or transcriptomic profile that represents the collective cell population. This approach enables the identification of common genetic variations and expression patterns but lacks resolution at the individual cell level. Bulk sequencing remains fundamental for detecting broad molecular changes in heterogeneous tissues before applying more detailed single-cell sequencing techniques.
Overview of Single-Cell Sequencing
Single-cell sequencing enables the analysis of genomic, transcriptomic, or epigenomic information at the resolution of individual cells, revealing cellular heterogeneity often masked in bulk sequencing methods. This technology allows for the identification of rare cell populations, gene expression variability, and dynamic cellular states within complex tissues. Advanced techniques such as single-cell RNA sequencing (scRNA-seq) facilitate high-throughput profiling, critical for understanding developmental processes, disease mechanisms, and personalized medicine applications.
Key Differences: Bulk vs Single-Cell Sequencing
Bulk sequencing analyzes DNA or RNA extracted from a mixture of cells, providing average genomic or transcriptomic profiles that mask cellular heterogeneity. Single-cell sequencing enables the examination of individual cells, revealing distinct genetic variations and expression patterns that bulk methods cannot detect. Key differences include resolution, with single-cell sequencing offering high spatial and temporal resolution, and data complexity, as it requires advanced computational methods to interpret cellular diversity.
Resolution and Sensitivity in Sequencing Methods
Sequencing methods vary significantly in resolution and sensitivity, with bulk sequencing providing average signals from mixed cell populations, thereby masking cellular heterogeneity. Single-cell sequencing achieves high resolution by analyzing genomic, transcriptomic, or epigenomic data at the individual cell level, revealing rare cell types and dynamic cellular states. Enhanced sensitivity in single-cell techniques allows detection of low-abundance transcripts and subtle genetic variations, crucial for understanding complex biological systems and disease mechanisms.
Applications in Genomics and Transcriptomics
Sequencing technologies enable comprehensive analysis of DNA or RNA to elucidate genomic variations and expression patterns, while single-cell sequencing provides granular insights into cellular heterogeneity by profiling individual cells. In genomics, traditional sequencing identifies bulk genetic mutations and structural variants, whereas single-cell sequencing reveals clonal evolution and rare subpopulations critical in cancer research. Transcriptomic applications leverage bulk RNA sequencing for average gene expression, but single-cell transcriptomics dissects cell-specific gene regulation and developmental trajectories at unprecedented resolution.
Advantages and Limitations of Each Approach
Sequencing techniques provide comprehensive insights into genetic material by analyzing bulk samples, allowing for high-throughput and cost-effective identification of genetic variants and expression patterns, but they often mask cellular heterogeneity due to averaging signals across populations. Single-cell sequencing offers granular resolution by profiling individual cells, enabling the detection of rare cell types, cellular heterogeneity, and dynamic states within tissues, though it faces challenges like higher costs, complex data analysis, and technical variability such as dropout events. Balancing sensitivity, resolution, and scalability is crucial when selecting between bulk sequencing and single-cell sequencing to address specific biological questions.
Data Analysis Challenges and Solutions
Sequencing data analysis faces challenges such as high dimensionality, noise, and large-scale data management, which are amplified in single-cell sequencing due to cell-to-cell variability and dropout events. Advanced algorithms like normalization methods, dimensionality reduction techniques (e.g., PCA, t-SNE, UMAP), and specialized machine learning approaches help mitigate data sparsity and heterogeneity in single-cell datasets. Integrating multi-omics data and leveraging cloud computing platforms enhances scalability and accuracy in interpreting complex single-cell sequencing results.
Emerging Trends in Single-Cell Sequencing
Emerging trends in single-cell sequencing highlight advancements such as spatial transcriptomics, which integrates gene expression data with cellular localization to provide a comprehensive tissue map. Improvements in multi-omics approaches facilitate simultaneous profiling of genomics, transcriptomics, and epigenomics at the single-cell level, enabling deeper insights into cellular heterogeneity and disease mechanisms. Enhanced bioinformatics tools and machine learning algorithms are revolutionizing data analysis, increasing resolution and accuracy in identifying rare cell populations and dynamic cellular states.
Future Directions in Sequencing Technologies
Future directions in sequencing technologies emphasize enhancing accuracy, speed, and cost-efficiency through innovations like nanopore and long-read sequencing platforms. Single-cell sequencing is advancing towards multi-omics integration, enabling simultaneous analysis of genomic, transcriptomic, and epigenomic data at the individual cell level. These developments aim to unravel complex biological systems and accelerate personalized medicine by providing unprecedented resolution and comprehensive molecular insights.
Related Important Terms
Spatial Transcriptomics
Sequencing analyzes bulk RNA to provide average gene expression profiles, while single-cell sequencing reveals transcriptional heterogeneity at the individual cell level, enabling detailed cellular characterization. Spatial transcriptomics integrates gene expression data with spatial information, mapping cellular functions within tissue architecture to advance understanding of tissue organization and cellular interactions.
Multi-omics Integration
Multi-omics integration in sequencing combines genomic, transcriptomic, epigenomic, and proteomic data to provide comprehensive insights into cellular functions, surpassing the resolution of traditional bulk sequencing. Single-cell sequencing enhances this approach by enabling multi-omics profiling at the individual cell level, revealing cellular heterogeneity and dynamic molecular interactions within complex tissues.
Pseudotime Trajectory Inference
Sequencing techniques provide bulk gene expression profiles, whereas single-cell sequencing captures transcriptomic heterogeneity at individual cell resolution, enabling precise mapping of cellular states. Pseudotime trajectory inference leverages single-cell RNA-seq data to reconstruct dynamic biological processes by ordering cells along a temporal continuum based on gene expression changes.
Single-Cell ATAC-seq
Single-Cell ATAC-seq enables high-resolution profiling of chromatin accessibility at the individual cell level, revealing cellular heterogeneity and regulatory element activity that bulk sequencing methods cannot detect. This technique provides insights into gene regulation dynamics, cell type-specific epigenomic landscapes, and developmental processes with unprecedented precision.
CITE-seq (Cellular Indexing of Transcriptomes and Epitopes)
CITE-seq integrates single-cell RNA sequencing with protein surface marker profiling, enabling simultaneous analysis of transcriptomes and epitopes at the individual cell level and providing a multidimensional perspective on cellular heterogeneity. This technique surpasses traditional sequencing by combining RNA and protein data, facilitating more precise cellular characterization and improved identification of immune cell subsets within complex tissues.
Index Hopping
Index hopping represents a significant challenge in both bulk sequencing and single-cell sequencing, causing misassignment of reads and potentially confounding data interpretation. Single-cell sequencing is particularly sensitive to index hopping due to the need for precise cell-specific barcoding, necessitating improved experimental designs and bioinformatics pipelines to accurately mitigate its impact.
Single-Nucleus Sequencing
Single-nucleus sequencing enables high-resolution analysis of gene expression and chromatin accessibility within individual nuclei, overcoming limitations of traditional single-cell sequencing in capturing complex tissues or archived samples where intact cells are difficult to isolate. This method provides critical insights into cellular heterogeneity and epigenomic regulation by preserving nuclear RNA and DNA, making it invaluable for neurosciences and disease research.
Barcode Swapping
Barcode swapping in standard sequencing causes misassignment of reads between samples, reducing data accuracy and leading to false variant calls. Single-cell sequencing employs unique molecular identifiers and cell-specific barcodes to minimize barcode swapping effects, ensuring higher resolution and precise cell lineage tracking.
Single-Cell CNV Profiling
Single-cell CNV profiling enables precise detection of copy number variations at an individual cell level, revealing intratumoral heterogeneity and clonal evolution that bulk sequencing methods cannot resolve. This high-resolution approach enhances understanding of cancer progression and therapeutic resistance by mapping genomic alterations within diverse cellular populations.
Duplex Sequencing
Duplex Sequencing enhances single-cell sequencing accuracy by independently tagging and analyzing both DNA strands to reduce sequencing errors and detect ultra-rare mutations. This method surpasses conventional sequencing by providing higher fidelity data critical for precise genomic analysis in cancer research and genetic disorder studies.
Sequencing vs Single-Cell Sequencing Infographic
 industrydif.com
industrydif.com