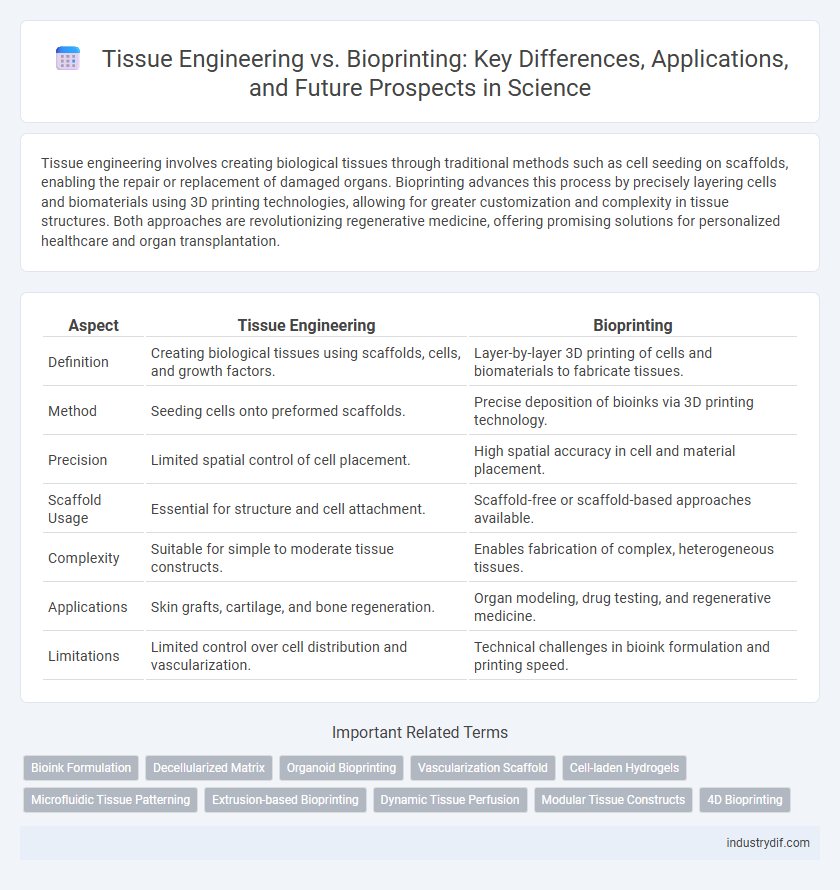
Tissue engineering involves creating biological tissues through traditional methods such as cell seeding on scaffolds, enabling the repair or replacement of damaged organs. Bioprinting advances this process by precisely layering cells and biomaterials using 3D printing technologies, allowing for greater customization and complexity in tissue structures. Both approaches are revolutionizing regenerative medicine, offering promising solutions for personalized healthcare and organ transplantation.
Table of Comparison
| Aspect | Tissue Engineering | Bioprinting |
|---|---|---|
| Definition | Creating biological tissues using scaffolds, cells, and growth factors. | Layer-by-layer 3D printing of cells and biomaterials to fabricate tissues. |
| Method | Seeding cells onto preformed scaffolds. | Precise deposition of bioinks via 3D printing technology. |
| Precision | Limited spatial control of cell placement. | High spatial accuracy in cell and material placement. |
| Scaffold Usage | Essential for structure and cell attachment. | Scaffold-free or scaffold-based approaches available. |
| Complexity | Suitable for simple to moderate tissue constructs. | Enables fabrication of complex, heterogeneous tissues. |
| Applications | Skin grafts, cartilage, and bone regeneration. | Organ modeling, drug testing, and regenerative medicine. |
| Limitations | Limited control over cell distribution and vascularization. | Technical challenges in bioink formulation and printing speed. |
Defining Tissue Engineering
Tissue engineering involves the multidisciplinary approach of combining scaffolds, cells, and biologically active molecules to develop functional tissues for medical applications. This field aims to restore, maintain, or improve damaged tissues or organs by mimicking the native extracellular matrix and promoting cell growth and differentiation. Unlike bioprinting, which uses layer-by-layer additive manufacturing technologies, tissue engineering relies more heavily on traditional scaffold fabrication and cell culture techniques.
Understanding Bioprinting Technologies
Bioprinting technologies utilize layer-by-layer deposition of bioinks containing living cells and biomaterials to create complex, functional tissue constructs with precise spatial organization. Unlike traditional tissue engineering, which relies on scaffold fabrication followed by cell seeding, bioprinting enables direct placement of multiple cell types and growth factors, enhancing tissue mimicry and vascularization. Advanced bioprinting modalities, such as inkjet, extrusion, and laser-assisted printing, offer varying resolutions and cell viability suited for different tissue engineering applications.
Historical Evolution: Tissue Engineering and Bioprinting
The historical evolution of tissue engineering began in the 1990s with the development of scaffold-based methods to regenerate tissues by combining cells, biomaterials, and growth factors. Bioprinting emerged in the early 2000s as an advanced technology leveraging 3D printing techniques to precisely position cells and biomaterials layer-by-layer, enhancing tissue complexity and functionality. Continuous innovations in biomaterials, stem cell biology, and printing technologies have propelled bioprinting to become a promising approach for fabricating complex tissue constructs beyond traditional tissue engineering capabilities.
Key Materials Used in Tissue Engineering
Tissue engineering primarily utilizes biomaterials such as natural polymers like collagen, gelatin, and alginate, as well as synthetic polymers including polyglycolic acid (PGA) and polylactic acid (PLA) to create scaffolds that support cell growth and differentiation. Bioprinting incorporates bioinks composed of hydrogels combined with living cells, extracellular matrix components, and growth factors to accurately fabricate 3D tissue constructs with spatial control over cellular organization. Both approaches rely heavily on materials that mimic native tissue environments, promoting cell viability and functional tissue regeneration.
Bioinks and Their Role in Bioprinting
Bioinks are critical in bioprinting as they provide the biological and mechanical support necessary for cell viability and tissue formation, combining biomaterials like hydrogels with living cells and growth factors. Tissue engineering traditionally uses scaffolds and cell seeding techniques, whereas bioprinting relies on the precise deposition of bioinks layer-by-layer to create complex, functional tissue constructs. Advances in bioink formulations, including the development of shear-thinning hydrogels and bioactive composites, enhance printability, structural integrity, and cellular responses crucial for replicating native tissue architecture.
Comparing Scaffold Design Techniques
Tissue engineering scaffold design primarily involves creating porous structures using materials like hydrogels, ceramics, and synthetic polymers to support cell attachment, proliferation, and differentiation. Bioprinting advances scaffold fabrication by enabling precise spatial deposition of biomaterials and living cells, facilitating complex, heterogeneous tissue architectures unattainable with traditional scaffold methods. Comparing these techniques reveals bioprinting's superior capability in replicating native tissue microenvironments through layer-by-layer customization and enhanced biomimicry.
Cell Sources and Manipulation Approaches
Tissue engineering primarily utilizes autologous, allogeneic, and stem cells, employing scaffolds and biochemical signals to guide cell growth and differentiation. Bioprinting advances this by leveraging precise spatial control of heterogeneous cell-laden bioinks, enabling layer-by-layer deposition to recreate complex tissue architectures. Both approaches manipulate cell phenotypes through mechanical, chemical, and genetic methods, but bioprinting offers enhanced customization in cell placement and microenvironment recreation.
Applications in Regenerative Medicine
Tissue engineering utilizes scaffolds, cells, and biologically active molecules to create functional tissues for regenerative medicine, enabling the repair or replacement of damaged organs such as skin, cartilage, and bone. Bioprinting advances this approach by precisely layering bioinks composed of living cells and biomaterials, facilitating the fabrication of complex, patient-specific tissues with enhanced structural fidelity. Applications in regenerative medicine include custom implants, wound healing, and organ regeneration, with bioprinting accelerating development through improved spatial control and scalability.
Current Limitations and Technical Challenges
Tissue engineering faces significant limitations in replicating complex tissue architectures due to inadequate vascularization and mechanical stability, hindering long-term functionality. Bioprinting struggles with challenges such as maintaining cell viability during the printing process, achieving high-resolution structures, and integrating multiple cell types with precise spatial organization. Both fields require advances in biomaterial development, scaffold design, and real-time monitoring techniques to overcome current technical barriers.
Future Trends in Tissue Engineering and Bioprinting
Future trends in tissue engineering emphasize the integration of advanced biomaterials with stem cell technology to enhance tissue regeneration and functionality. Bioprinting developments focus on increasing resolution and vascularization within printed constructs to enable the creation of viable, complex organs for transplantation. Emerging approaches combine AI-driven modeling with bioprinting to optimize scaffold design and accelerate personalized therapeutic applications.
Related Important Terms
Bioink Formulation
Bioink formulation in tissue engineering integrates biomaterials, living cells, and bioactive molecules to create a viable scaffold that supports cellular growth and differentiation, optimizing mechanical properties and biocompatibility for precise tissue regeneration. Advances in bioprinting technology enable the customization of bioinks with tunable viscosity and crosslinking mechanisms, enhancing printability, structural fidelity, and functional integration in complex tissue constructs.
Decellularized Matrix
Decellularized matrix offers a biologically native scaffold with preserved extracellular matrix components essential for cell adhesion and differentiation, making it a superior choice in tissue engineering frameworks. Bioprinting leverages this matrix to enhance spatial precision and complexity in constructing tissue-like structures with viable cell integration.
Organoid Bioprinting
Organoid bioprinting integrates advanced 3D bioprinting techniques with tissue engineering to fabricate complex, functional mini-organs that replicate native tissue architecture and cellular heterogeneity. This method surpasses traditional tissue engineering by enhancing spatial control over cellular placement, enabling precise vascularization, and improving the scalability and reproducibility of organoid construction for regenerative medicine and disease modeling.
Vascularization Scaffold
Tissue engineering utilizes vascularization scaffolds designed with porous architectures to promote nutrient diffusion and capillary growth, optimizing tissue regeneration. Bioprinting advances this by precisely depositing bioinks containing endothelial cells and growth factors, enabling the fabrication of complex vascular networks essential for functional tissue constructs.
Cell-laden Hydrogels
Cell-laden hydrogels serve as critical scaffolds in tissue engineering by providing a biocompatible matrix that supports cell viability, proliferation, and differentiation for tissue regeneration. In bioprinting, these hydrogels function as bioinks that enable precise spatial organization of multiple cell types, enhancing the fabrication of complex tissue constructs with controlled microarchitecture.
Microfluidic Tissue Patterning
Microfluidic tissue patterning enables precise spatial control of cell and extracellular matrix deposition, enhancing the complexity and functionality of engineered tissues beyond traditional tissue engineering methods. Bioprinting integrates microfluidic technologies to fabricate heterogeneous tissue constructs with vascular-like networks, improving cell viability and mimicking native tissue architecture at microscale resolutions.
Extrusion-based Bioprinting
Extrusion-based bioprinting enables precise layering of cell-laden bioinks to create complex tissue constructs, offering enhanced control over scaffold architecture compared to traditional tissue engineering methods. This technique facilitates high cell viability and spatial heterogeneity, crucial for replicating native tissue microenvironments in regenerative medicine applications.
Dynamic Tissue Perfusion
Dynamic tissue perfusion in tissue engineering enhances nutrient and oxygen delivery through bioreactor systems, promoting cell viability and tissue maturation. Bioprinting integrates dynamic perfusion channels within printed constructs, enabling precise vascularization and improved functional tissue development.
Modular Tissue Constructs
Modular tissue constructs in tissue engineering involve assembling small, functional tissue units that promote improved cellular organization and vascularization, enhancing tissue regeneration and scalability. Bioprinting advances this approach by precisely depositing biomaterials and living cells layer-by-layer, enabling the fabrication of complex, heterogeneous tissue architectures with high spatial resolution.
4D Bioprinting
Tissue engineering involves creating functional biological tissues by combining cells, scaffolds, and bioactive molecules, while bioprinting specifically utilizes layer-by-layer deposition of bioinks to fabricate complex structures. 4D bioprinting advances this technology by integrating time as a dynamic factor, enabling printed tissues to undergo predefined transformations in shape or function in response to environmental stimuli, thereby enhancing tissue maturation and adaptability.
Tissue Engineering vs Bioprinting Infographic
 industrydif.com
industrydif.com