DNA sequencing traditionally relies on methods like Sanger sequencing, offering accurate but time-consuming analysis of genetic material. Nanopore sequencing represents a breakthrough technology that enables real-time, portable, and long-read sequencing by detecting changes in electrical current as DNA strands pass through nanopores. This method allows for rapid identification of complex genetic variations and epigenetic modifications, making it valuable for scientific research and clinical applications.
Table of Comparison
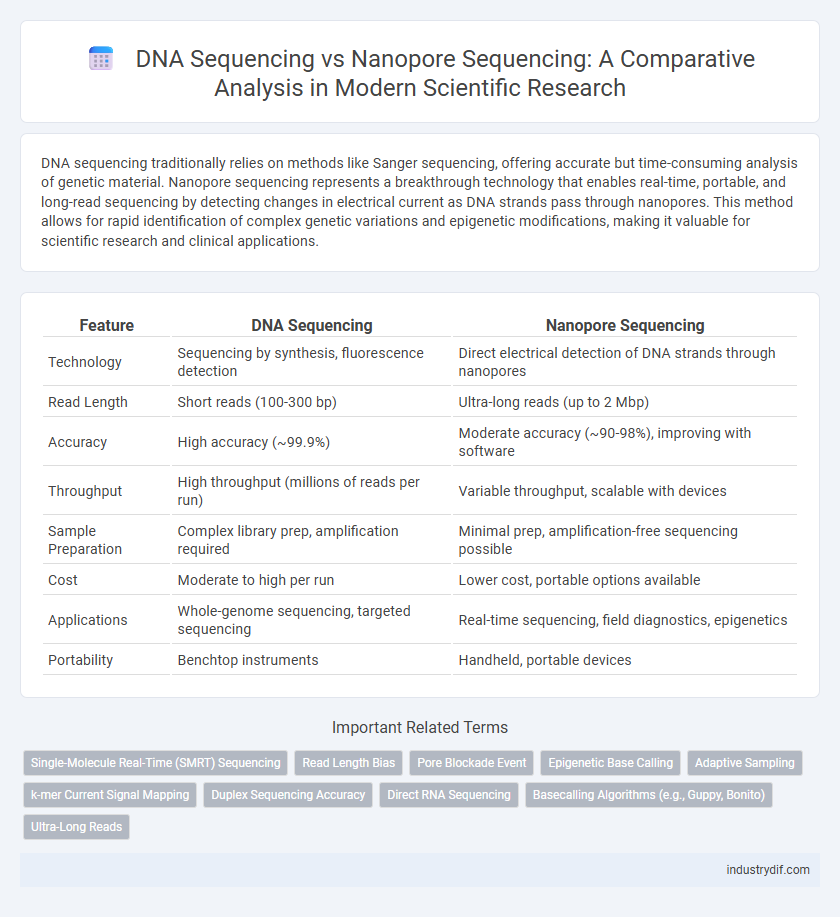
| Feature | DNA Sequencing | Nanopore Sequencing |
|---|---|---|
| Technology | Sequencing by synthesis, fluorescence detection | Direct electrical detection of DNA strands through nanopores |
| Read Length | Short reads (100-300 bp) | Ultra-long reads (up to 2 Mbp) |
| Accuracy | High accuracy (~99.9%) | Moderate accuracy (~90-98%), improving with software |
| Throughput | High throughput (millions of reads per run) | Variable throughput, scalable with devices |
| Sample Preparation | Complex library prep, amplification required | Minimal prep, amplification-free sequencing possible |
| Cost | Moderate to high per run | Lower cost, portable options available |
| Applications | Whole-genome sequencing, targeted sequencing | Real-time sequencing, field diagnostics, epigenetics |
| Portability | Benchtop instruments | Handheld, portable devices |
Introduction to DNA Sequencing Technologies
DNA sequencing technologies have evolved from traditional Sanger sequencing to advanced methods like nanopore sequencing, which offers real-time, long-read capabilities. Nanopore sequencing enables direct analysis of single DNA molecules without amplification, providing advantages in speed and read length over short-read methods. These innovations are critical for applications in genomics, personalized medicine, and complex genome assembly.
Overview of Traditional DNA Sequencing Methods
Traditional DNA sequencing methods, such as Sanger sequencing and Illumina sequencing, rely on chain termination or sequencing-by-synthesis techniques to determine nucleotide sequences with high accuracy. Sanger sequencing, known for its long read lengths and low error rates, remains a gold standard for small-scale projects and validation. Illumina sequencing offers massively parallel short reads, enabling high throughput and cost-effective analysis but often requires complex assembly for genome reconstruction.
What is Nanopore Sequencing?
Nanopore sequencing is a cutting-edge DNA sequencing technology that reads nucleotide sequences by detecting changes in electrical current as single DNA molecules pass through a nanoscale pore. This method enables real-time, long-read sequencing with high accuracy and minimal sample preparation, making it advantageous over traditional short-read sequencing techniques. Its portability and rapid data generation revolutionize genomic analyses in clinical diagnostics, environmental monitoring, and personalized medicine.
Comparative Workflow: Sanger, NGS, and Nanopore
Sanger sequencing involves chain termination and capillary electrophoresis, producing high-accuracy but low-throughput data suitable for small-scale projects. Next-generation sequencing (NGS) platforms use massively parallel sequencing with short reads, enabling high-throughput and cost-effective analysis of complex genomes. Nanopore sequencing differentiates itself by enabling real-time, long-read sequencing without amplification, offering advantages in identifying structural variations and epigenetic modifications.
Accuracy and Read Lengths: Head-to-Head
DNA sequencing technologies vary significantly in accuracy and read lengths, with traditional methods like Illumina offering high accuracy but limited read lengths up to 300 base pairs. Nanopore sequencing provides ultra-long reads exceeding hundreds of kilobases, enabling comprehensive genome assemblies despite slightly lower raw read accuracy around 90-95%. Error correction algorithms and consensus sequencing improve nanopore accuracy, making it increasingly competitive for applications requiring both long reads and reliable base calling.
Sample Preparation: Requirements and Challenges
DNA sequencing techniques differ significantly in sample preparation, with traditional methods often requiring extensive DNA extraction, amplification, and fragmentation to obtain high-quality templates. Nanopore sequencing streamlines sample preparation by allowing direct analysis of native DNA or RNA molecules, reducing the need for complex preprocessing steps, yet it demands careful handling to maintain molecule integrity and avoid contamination. Challenges in nanopore sequencing include minimizing sample degradation and optimizing buffer conditions to enhance pore stability and signal accuracy during real-time sequencing.
Throughput and Scalability in Modern Sequencing
DNA sequencing encompasses various methods, with nanopore sequencing standing out for its high throughput and scalability in modern genomic analysis. Nanopore technology enables real-time, long-read sequencing with scalable platform sizes, accommodating both small-scale research and large-scale population genomics projects. This contrasts with traditional sequencing techniques, which often require extensive sample preparation and have limited throughput, impeding rapid data generation for extensive genomic studies.
Cost Analysis: Investment and Running Expenses
Nanopore sequencing offers lower initial investment compared to traditional DNA sequencing platforms, reducing entry barriers for smaller laboratories. Running expenses for nanopore sequencing are minimized by the reusable nature of flow cells and reduced reagent consumption, making long-term operational costs more economical. Traditional DNA sequencing involves higher capital costs and ongoing maintenance fees, often resulting in greater total expenditure for equivalent throughput.
Applications in Genomics, Diagnostics, and Research
DNA sequencing techniques, including traditional methods and nanopore sequencing, serve crucial roles in genomics, diagnostics, and research by enabling comprehensive analysis of genetic material. Nanopore sequencing distinguishes itself with real-time data generation and long-read capabilities, facilitating detailed genomic mapping, rapid pathogen identification, and advanced studies in transcriptomics and epigenetics. This technology propels precision medicine by enhancing diagnostic accuracy and supporting personalized treatment strategies through direct detection of genetic variations and modifications.
Future Trends in Sequencing Technology and Innovation
Nanopore sequencing is rapidly advancing as a transformative technology, offering real-time, long-read capabilities that surpass traditional DNA sequencing methods limited by short-read lengths and complex sample preparation. Emerging trends include increasing accuracy through improved nanopore chemistry and AI-driven basecalling algorithms, enabling more precise detection of epigenetic modifications and structural variants. Integration of portable nanopore devices with cloud-based analytics is anticipated to democratize genomic research and accelerate personalized medicine applications.
Related Important Terms
Single-Molecule Real-Time (SMRT) Sequencing
Single-Molecule Real-Time (SMRT) sequencing offers long-read DNA sequencing capabilities by directly observing DNA polymerase activity, enabling high-resolution detection of epigenetic modifications and complex genomic regions missed by traditional short-read nanopore sequencing. SMRT sequencing provides higher accuracy and consensus quality through circular consensus sequencing, making it ideal for de novo genome assembly and structural variation analysis in comparison to nanopore platforms.
Read Length Bias
DNA sequencing techniques exhibit significant variations in read length bias, with traditional methods like Illumina producing short reads typically under 300 base pairs, while nanopore sequencing can generate ultra-long reads exceeding 100,000 base pairs. This disparity impacts genomic assembly and variant detection, as nanopore sequencing's extended read length reduces bias by spanning repetitive regions and structural variants more effectively than short-read technologies.
Pore Blockade Event
DNA sequencing traditionally relies on amplification and fluorescence signals, whereas nanopore sequencing identifies nucleotides through characteristic pore blockade events caused by DNA strands passing in real-time. The duration and magnitude of ionic current disruptions during these blockade events provide direct, label-free insights into the nucleotide sequence and epigenetic modifications.
Epigenetic Base Calling
Nanopore sequencing enhances epigenetic base calling by directly detecting DNA modifications such as methylation, unlike traditional DNA sequencing methods that require bisulfite conversion or indirect inference. This real-time, label-free approach allows for more accurate and comprehensive mapping of epigenetic markers, facilitating advanced studies in gene regulation and disease mechanisms.
Adaptive Sampling
DNA sequencing methods differ significantly in adaptive sampling capabilities, with nanopore sequencing offering real-time selective sequencing by dynamically enriching target sequences through voltage modulation, enhancing genomic coverage efficiency. This contrasts with traditional sequencing platforms, which lack real-time feedback and require prior sample enrichment, limiting their adaptability during sequencing runs.
k-mer Current Signal Mapping
K-mer current signal mapping in nanopore sequencing enhances real-time DNA sequence analysis by directly correlating characteristic ionic current disruptions with specific nucleotide sequences, enabling higher resolution and accuracy compared to traditional DNA sequencing methods. This approach leverages the distinct electrical signatures of overlapping k-mers passing through nanopores, facilitating rapid base-calling and improved detection of sequence variants and epigenetic modifications.
Duplex Sequencing Accuracy
Duplex sequencing enhances DNA sequencing accuracy by analyzing both strands of the DNA molecule, significantly reducing errors compared to traditional Nanopore sequencing methods. This approach achieves ultra-high fidelity by cross-verifying complementary strand information, enabling precise detection of rare mutations in genomic studies.
Direct RNA Sequencing
Direct RNA sequencing via nanopore technology enables real-time analysis of native RNA molecules without the need for reverse transcription or amplification, preserving nucleotide modifications and structural information. Unlike traditional DNA sequencing methods, nanopore sequencing offers long reads and direct observation of RNA epigenetic marks, enhancing transcriptomic studies and functional genomics research.
Basecalling Algorithms (e.g., Guppy, Bonito)
Basecalling algorithms like Guppy and Bonito are critical in nanopore sequencing for translating raw electrical signals into nucleotide sequences, offering enhanced accuracy compared to traditional DNA sequencing methods. Guppy utilizes a neural network model optimized for speed and precision, while Bonito employs deep learning frameworks to improve basecalling accuracy, enabling real-time data analysis and superior detection of epigenetic modifications.
Ultra-Long Reads
Nanopore sequencing enables ultra-long DNA reads exceeding 2 megabases, surpassing traditional sequencing methods limited to shorter fragments under 300 kilobases, thereby enhancing genomic assembly accuracy and structural variation detection. This technology leverages real-time electrical signal measurement as DNA strands pass through nanopores, facilitating high-throughput, single-molecule analysis without PCR amplification.
DNA Sequencing vs Nanopore Sequencing Infographic
 industrydif.com
industrydif.com