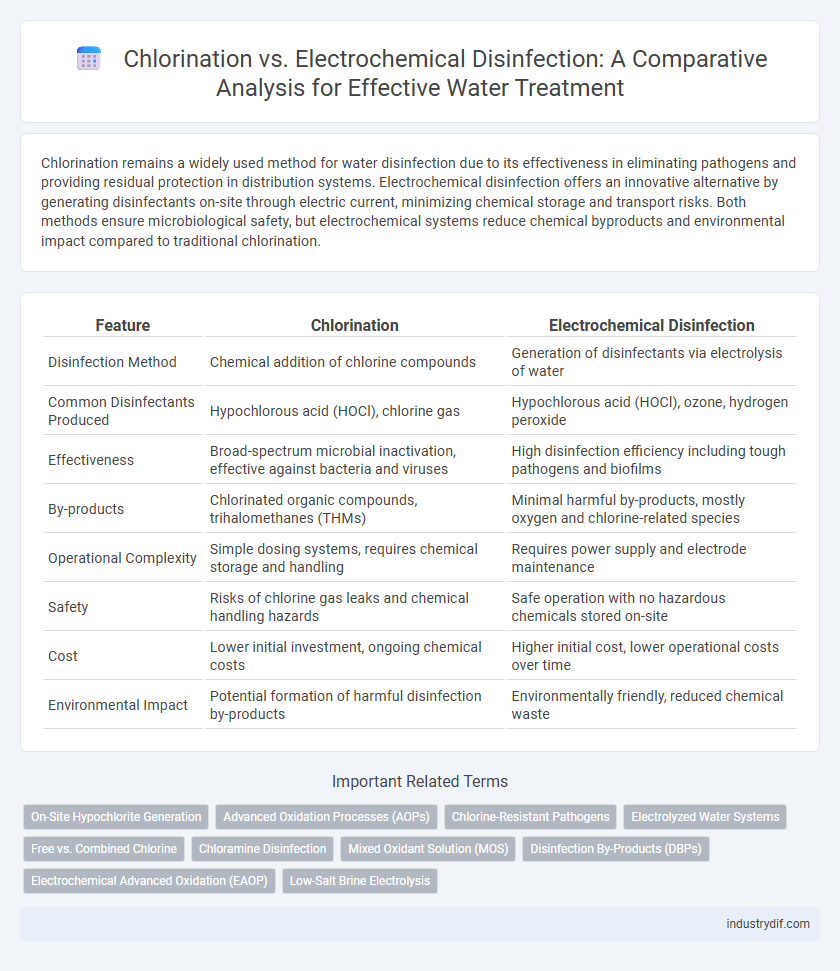
Chlorination remains a widely used method for water disinfection due to its effectiveness in eliminating pathogens and providing residual protection in distribution systems. Electrochemical disinfection offers an innovative alternative by generating disinfectants on-site through electric current, minimizing chemical storage and transport risks. Both methods ensure microbiological safety, but electrochemical systems reduce chemical byproducts and environmental impact compared to traditional chlorination.
Table of Comparison
| Feature | Chlorination | Electrochemical Disinfection |
|---|---|---|
| Disinfection Method | Chemical addition of chlorine compounds | Generation of disinfectants via electrolysis of water |
| Common Disinfectants Produced | Hypochlorous acid (HOCl), chlorine gas | Hypochlorous acid (HOCl), ozone, hydrogen peroxide |
| Effectiveness | Broad-spectrum microbial inactivation, effective against bacteria and viruses | High disinfection efficiency including tough pathogens and biofilms |
| By-products | Chlorinated organic compounds, trihalomethanes (THMs) | Minimal harmful by-products, mostly oxygen and chlorine-related species |
| Operational Complexity | Simple dosing systems, requires chemical storage and handling | Requires power supply and electrode maintenance |
| Safety | Risks of chlorine gas leaks and chemical handling hazards | Safe operation with no hazardous chemicals stored on-site |
| Cost | Lower initial investment, ongoing chemical costs | Higher initial cost, lower operational costs over time |
| Environmental Impact | Potential formation of harmful disinfection by-products | Environmentally friendly, reduced chemical waste |
Overview of Water Disinfection Methods
Chlorination and electrochemical disinfection are prominent methods used for water treatment, each offering distinct advantages in pathogen removal efficiency. Chlorination, widely adopted for its cost-effectiveness and residual disinfectant properties, effectively eliminates bacteria and viruses but may produce disinfection byproducts like trihalomethanes. Electrochemical disinfection employs electrical current to generate oxidants such as chlorine on-site, minimizing chemical storage and reducing harmful byproducts, making it suitable for decentralized water systems and point-of-use applications.
Understanding Chlorination: Principles and Process
Chlorination involves adding chlorine or chlorine compounds to water to eliminate pathogens through oxidation and disruption of cellular functions. The process relies on forming hypochlorous acid (HOCl), a potent disinfectant that penetrates microbial cell walls and inactivates enzymes critical for survival. This method remains a widely used, cost-effective solution for ensuring potable water safety and controlling waterborne diseases.
What is Electrochemical Disinfection?
Electrochemical disinfection is a water treatment process that generates disinfectants through the electrolysis of saline water, producing reactive species such as chlorine, hypochlorous acid, and hydroxyl radicals. This method offers on-site generation of disinfectants, reducing the need for chemical storage and transport, while effectively inactivating bacteria, viruses, and protozoa. Electrochemical disinfection systems provide a sustainable and efficient alternative to traditional chlorination by minimizing harmful disinfection byproducts and allowing precise control of disinfection levels.
Key Differences between Chlorination and Electrochemical Disinfection
Chlorination involves adding chemical chlorine compounds to water to eliminate pathogens, whereas electrochemical disinfection generates disinfectant agents in situ through electrical currents applied to electrodes immersed in water. Chlorination is widely used due to its residual disinfection capability and low cost, but it can produce harmful disinfection byproducts such as trihalomethanes. Electrochemical disinfection offers on-demand production of disinfectants with lower chemical handling risks and reduced formation of hazardous byproducts, although it typically requires higher energy input and advanced system maintenance.
Efficacy Against Waterborne Pathogens
Chlorination effectively eliminates a broad spectrum of waterborne pathogens, including bacteria, viruses, and protozoa, with proven residual disinfectant properties to prevent recontamination. Electrochemical disinfection generates reactive species such as chlorine and hydroxyl radicals on-site, ensuring rapid inactivation of pathogens and reducing chemical handling risks. Both methods demonstrate high efficacy, but electrochemical disinfection offers enhanced control over disinfection byproducts and improved safety for potable water treatment.
Chemical Byproducts and Safety Concerns
Chlorination produces chemical byproducts such as trihalomethanes (THMs) and haloacetic acids (HAAs), which pose health risks including cancer and reproductive issues. Electrochemical disinfection generates fewer harmful byproducts, primarily releasing chloride ions and minimal chlorate or perchlorate, enhancing safety for drinking water. Both methods require careful monitoring, but electrochemical disinfection offers a reduced chemical byproduct profile, improving overall water treatment safety.
Operational Costs and Maintenance Requirements
Chlorination typically incurs lower upfront operational costs but demands continuous chemical supply and handling, increasing long-term expenses and safety concerns. Electrochemical disinfection requires higher initial investment and energy consumption but offers reduced chemical storage, simpler dosing, and lower maintenance complexity. Facilities prioritize electrochemical systems to minimize hazardous chemical management and achieve consistent disinfection with lower operator intervention.
Environmental Impact Assessment
Chlorination produces harmful byproducts such as trihalomethanes and chloramines, contributing to water toxicity and posing risks to aquatic ecosystems. Electrochemical disinfection offers a greener alternative, minimizing chemical residuals and reducing the formation of toxic compounds, thereby lowering environmental footprints. Life cycle assessments indicate electrochemical methods consume less energy and generate fewer pollutants compared to conventional chlorination processes.
Applications in Industrial Water Treatment
Chlorination remains a widely used method for industrial water treatment due to its effectiveness in microbial control and cost-efficiency, particularly in large-scale facilities such as cooling towers and wastewater plants. Electrochemical disinfection offers a chemical-free alternative by generating disinfectants on-site through electrolysis, enhancing safety and reducing chemical storage risks in industries like food processing and pharmaceuticals. Both methods achieve regulatory compliance, but electrochemical systems provide better integration with automated control systems for continuous water quality monitoring.
Future Trends in Water Disinfection Technologies
Future trends in water disinfection technologies emphasize the growing adoption of electrochemical disinfection due to its efficiency in generating reactive species like hydroxyl radicals, which effectively inactivate pathogens without harmful byproducts. Chlorination remains widely used for its cost-effectiveness and residual disinfection properties but faces challenges related to disinfection byproducts such as trihalomethanes. Advances in membrane electrolysis and electrode materials are enhancing the scalability and sustainability of electrochemical methods, positioning them as promising solutions for next-generation water treatment systems.
Related Important Terms
On-Site Hypochlorite Generation
On-site hypochlorite generation provides a safer, cost-effective alternative to traditional chlorination by producing sodium hypochlorite on demand through electrochemical disinfection, eliminating the need for hazardous chemical storage and transportation. This method enhances water treatment efficiency and sustainability by offering precise dosing, reduced chemical waste, and improved control over disinfection processes in municipal and industrial water systems.
Advanced Oxidation Processes (AOPs)
Chlorination relies on chlorine-based chemicals to disinfect water, effectively targeting a wide range of pathogens but often producing harmful disinfection byproducts such as trihalomethanes. Electrochemical disinfection using Advanced Oxidation Processes (AOPs) generates powerful oxidants like hydroxyl radicals in situ, offering superior degradation of organic contaminants and enhanced microbial inactivation with minimal hazardous residuals.
Chlorine-Resistant Pathogens
Chlorination effectively eliminates many waterborne pathogens but struggles against chlorine-resistant pathogens such as Cryptosporidium and Giardia, which can survive standard chlorine levels. Electrochemical disinfection offers enhanced inactivation of these resistant microorganisms by generating multiple reactive species, providing a more robust barrier against chlorine-resistant waterborne diseases.
Electrolyzed Water Systems
Electrolyzed water systems generate powerful oxidants through electrochemical processes, effectively disinfecting water without harmful chemical residues. These systems offer a sustainable alternative to chlorination, reducing reliance on chlorine and minimizing the formation of disinfection by-products such as trihalomethanes.
Free vs. Combined Chlorine
Free chlorine, primarily in the form of hypochlorous acid (HOCl) and hypochlorite ion (OCl-), offers strong oxidizing properties for effective microbial inactivation in water treatment, while combined chlorine, mainly chloramines, provides longer-lasting residual disinfection but with reduced potency and potential taste and odor issues. Electrochemical disinfection generates free chlorine in situ from chloride ions, allowing precise control of free chlorine levels without forming chloramine byproducts, enhancing both efficiency and safety in potable water systems.
Chloramine Disinfection
Chloramine disinfection offers a more stable and longer-lasting residual effect compared to traditional chlorination, reducing the formation of harmful disinfection byproducts such as trihalomethanes. Electrochemical disinfection, while effective, often lacks the persistent disinfection qualities of chloramines, making chloramine a preferred choice for maintaining long distribution system integrity in water treatment.
Mixed Oxidant Solution (MOS)
Mixed Oxidant Solution (MOS) generated through electrochemical disinfection offers enhanced microbial control and reduced formation of harmful disinfection byproducts compared to traditional chlorination methods. MOS combines chlorine species and other reactive oxidants, providing longer-lasting residuals and improved efficacy against biofilms and resistant pathogens in water treatment.
Disinfection By-Products (DBPs)
Chlorination commonly produces disinfection by-products (DBPs) such as trihalomethanes (THMs) and haloacetic acids (HAAs), which pose health risks including carcinogenicity. Electrochemical disinfection significantly reduces the formation of harmful DBPs by generating reactive oxygen species on-site without adding chemical disinfectants, offering a safer alternative for water treatment.
Electrochemical Advanced Oxidation (EAOP)
Electrochemical Advanced Oxidation Processes (EAOP) generate highly reactive hydroxyl radicals that effectively degrade organic contaminants and pathogens without forming harmful disinfection by-products, unlike chlorination which often produces chlorinated compounds posing health risks. EAOP systems offer higher disinfection efficiency and environmental compatibility in water treatment by leveraging in-situ generation of oxidants such as hydroxyl radicals and ozone through electrochemical reactions.
Low-Salt Brine Electrolysis
Low-salt brine electrolysis offers an energy-efficient and environmentally friendly alternative to traditional chlorination by generating disinfectants on-site with minimal chemical handling and reduced salt concentrations, lowering both operational costs and corrosion risks. This electrochemical disinfection method produces hypochlorous acid directly in water, ensuring effective microbial control while minimizing harmful disinfection by-products often associated with conventional chlorination processes.
Chlorination vs Electrochemical Disinfection Infographic
 industrydif.com
industrydif.com