Chlorination remains a widely used water disinfection method due to its efficiency and cost-effectiveness, effectively eliminating many pathogens. Advanced Oxidation Processes (AOPs) utilize powerful oxidants like hydroxyl radicals to degrade complex organic contaminants and emerging pollutants that chlorination cannot fully remove. Combining both methods can enhance water quality by providing robust microbial inactivation alongside superior removal of recalcitrant compounds.
Table of Comparison
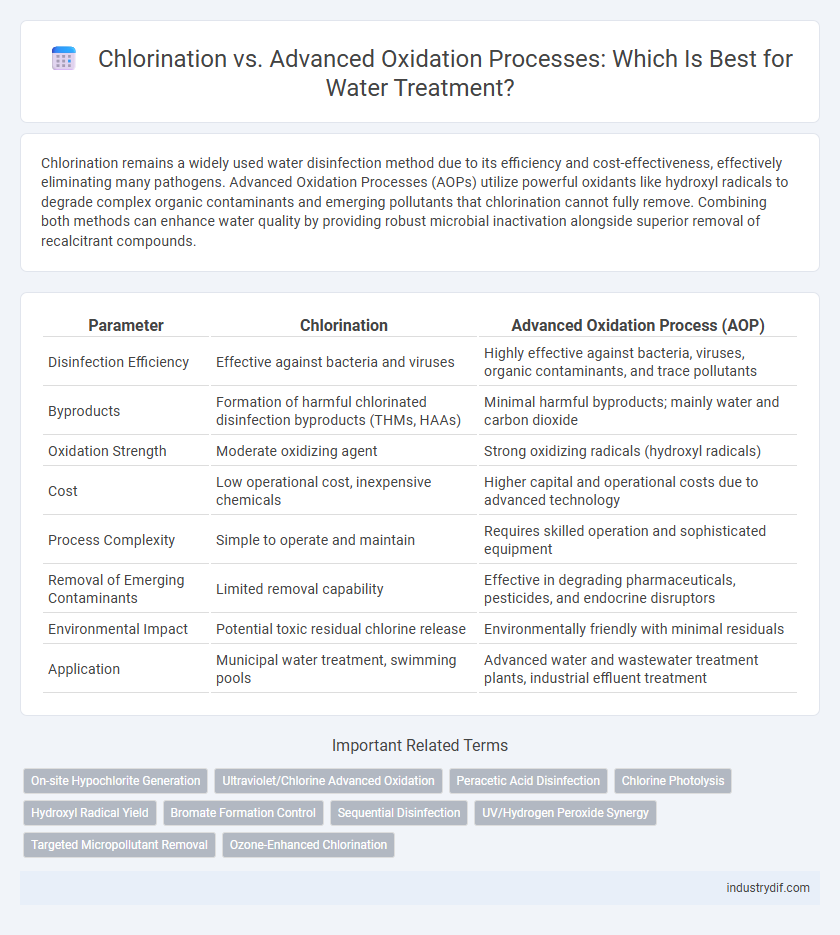
| Parameter | Chlorination | Advanced Oxidation Process (AOP) |
|---|---|---|
| Disinfection Efficiency | Effective against bacteria and viruses | Highly effective against bacteria, viruses, organic contaminants, and trace pollutants |
| Byproducts | Formation of harmful chlorinated disinfection byproducts (THMs, HAAs) | Minimal harmful byproducts; mainly water and carbon dioxide |
| Oxidation Strength | Moderate oxidizing agent | Strong oxidizing radicals (hydroxyl radicals) |
| Cost | Low operational cost, inexpensive chemicals | Higher capital and operational costs due to advanced technology |
| Process Complexity | Simple to operate and maintain | Requires skilled operation and sophisticated equipment |
| Removal of Emerging Contaminants | Limited removal capability | Effective in degrading pharmaceuticals, pesticides, and endocrine disruptors |
| Environmental Impact | Potential toxic residual chlorine release | Environmentally friendly with minimal residuals |
| Application | Municipal water treatment, swimming pools | Advanced water and wastewater treatment plants, industrial effluent treatment |
Introduction to Water Treatment Methods
Chlorination and Advanced Oxidation Process (AOP) are crucial water treatment methods used to eliminate contaminants and pathogens. Chlorination relies on chlorine compounds to disinfect water by killing bacteria and viruses, making it widely adopted for municipal water supplies. AOP employs reactive species such as hydroxyl radicals generated through processes involving ozone, hydrogen peroxide, or UV light to break down complex organic pollutants and ensure higher water quality.
Understanding Chlorination in Water Treatment
Chlorination remains a widely used water treatment method due to its effective disinfection properties and ability to maintain residual chlorine levels, ensuring microbial safety throughout the distribution system. It efficiently targets bacteria, viruses, and protozoa by disrupting cellular functions, making it a cost-effective and reliable solution for potable water treatment. However, chlorination can produce disinfection byproducts such as trihalomethanes (THMs) and haloacetic acids (HAAs), which require careful monitoring and regulation to minimize health risks.
Overview of Advanced Oxidation Processes (AOPs)
Advanced Oxidation Processes (AOPs) utilize reactive species such as hydroxyl radicals to effectively degrade organic contaminants and disinfect water. These processes include techniques like ozonation, Fenton reaction, and UV/H2O2 treatment that enhance pollutant mineralization beyond conventional chlorination. AOPs are increasingly favored for their ability to remove a broad spectrum of micropollutants and reduce disinfection byproducts compared to traditional chlorination methods.
Mechanisms of Disinfection: Chlorination vs. AOPs
Chlorination disinfects water by releasing free chlorine radicals that penetrate microbial cell walls, disrupting vital cellular functions and causing irreversible damage to pathogens. Advanced Oxidation Processes (AOPs) generate highly reactive hydroxyl radicals through reactions involving ozone, hydrogen peroxide, or UV light, which aggressively oxidize organic contaminants and microbial cell components at a molecular level. The distinct difference lies in chlorination targeting pathogen structures primarily through chlorinated compounds, while AOPs induce broad-spectrum oxidation capable of decomposing complex pollutants and microorganisms simultaneously.
Effectiveness Against Pathogens and Contaminants
Chlorination effectively eliminates a broad spectrum of pathogens, including bacteria and viruses, by disrupting cellular function through oxidation. Advanced Oxidation Processes (AOPs) generate highly reactive hydroxyl radicals that degrade complex organic contaminants and emerging pollutants more efficiently than chlorination. While chlorination provides residual disinfection in distribution systems, AOPs excel at removing micropollutants and resistant microorganisms, making them ideal for advanced water treatment applications.
Byproducts and Residuals: Safety Considerations
Chlorination in water treatment generates disinfection byproducts (DBPs) such as trihalomethanes (THMs) and haloacetic acids (HAAs), which pose health risks and require careful monitoring to ensure safety. Advanced Oxidation Processes (AOPs) produce fewer harmful byproducts and residuals, mainly breaking down contaminants into harmless end products like water and carbon dioxide. Safety considerations emphasize minimizing toxic residues and ensuring treated water complies with regulatory standards to protect public health.
Operational Costs and Energy Requirements
Chlorination typically involves lower operational costs and energy requirements compared to Advanced Oxidation Processes (AOPs), which rely heavily on energy-intensive UV light or ozone generation. AOPs demand higher electricity consumption and more complex maintenance, driving up overall expenses despite their superior contaminant removal efficiency. In contrast, chlorination's simpler infrastructure and chemical dosing system make it more cost-effective for large-scale water treatment facilities.
Environmental Impact of Chlorination and AOPs
Chlorination, a common water disinfection method, produces harmful disinfection byproducts (DBPs) like trihalomethanes and haloacetic acids, which pose risks to aquatic ecosystems and human health. Advanced Oxidation Processes (AOPs) generate fewer toxic byproducts by using hydroxyl radicals to degrade contaminants, resulting in less environmental pollution and improved biodegradability of treated water. Compared to chlorination, AOPs offer a more sustainable solution by minimizing persistent organic pollutants and reducing downstream ecological toxicity.
Regulatory Standards and Compliance
Chlorination remains a widely accepted water disinfection method governed by well-established regulatory standards such as the U.S. EPA Maximum Residual Disinfectant Level (MRDL) for chlorine at 4 mg/L, ensuring microbial safety and residual control. Advanced Oxidation Processes (AOPs), including ozone and UV/hydrogen peroxide, often face evolving regulatory frameworks due to their complex reaction by-products and variable efficacy, requiring compliance with stringent limits on disinfection by-products (DBPs) like bromate and formaldehyde under EPA and WHO guidelines. Water utilities must balance regulatory compliance for microbial inactivation, disinfection by-product formation, and operational feasibility when choosing between chlorination and AOP technologies.
Application Scenarios: Choosing the Right Technology
Chlorination is widely used for disinfecting drinking water and large-scale municipal wastewater treatment due to its cost-effectiveness and residual disinfection ability. Advanced Oxidation Process (AOP) suits applications requiring the removal of complex organic contaminants, pharmaceuticals, and micropollutants in industrial wastewater and surface water treatment. Selecting the appropriate technology depends on water quality goals, contaminant types, treatment scale, and regulatory compliance requirements.
Related Important Terms
On-site Hypochlorite Generation
On-site hypochlorite generation offers a safer and more cost-effective alternative to traditional chlorination by producing chlorine on demand, reducing storage risks and transportation costs. Advanced Oxidation Processes (AOPs) provide higher efficiency in degrading complex organic contaminants but often require higher energy input and complex operational control compared to on-site hypochlorite systems.
Ultraviolet/Chlorine Advanced Oxidation
Ultraviolet/Chlorine Advanced Oxidation Process (UV/Cl AOP) generates highly reactive hydroxyl radicals that effectively degrade micropollutants and disinfection byproducts, outperforming traditional chlorination in water treatment. UV/Cl AOP enhances pathogen inactivation and reduces harmful chlorinated compounds, offering a sustainable alternative for safer and cleaner water supply.
Peracetic Acid Disinfection
Peracetic acid disinfection in water treatment offers a potent alternative to chlorination by providing rapid microbial inactivation without forming harmful chlorinated byproducts, making it favorable for applications requiring safe and environmentally friendly solutions. Its strong oxidizing capability targets a broad spectrum of pathogens while decomposing into non-toxic residues, enhancing the sustainability of advanced oxidation processes in drinking water and wastewater treatment.
Chlorine Photolysis
Chlorine photolysis enhances traditional chlorination by using ultraviolet light to break down chlorine molecules, generating highly reactive hydroxyl radicals that improve the degradation of organic pollutants in water. This advanced approach increases disinfection efficiency and reduces harmful disinfection byproducts compared to conventional chlorination methods.
Hydroxyl Radical Yield
Chlorination produces lower hydroxyl radical yields compared to Advanced Oxidation Processes (AOPs), which generate significantly higher concentrations of hydroxyl radicals responsible for effective pollutant degradation. AOPs utilize hydroxyl radicals with oxidation potentials around 2.8 V, enhancing contaminant removal efficiency beyond the disinfection capabilities of chlorine-based treatments.
Bromate Formation Control
Chlorination effectively disinfects water but can produce harmful bromate byproducts, especially in bromide-rich sources. Advanced Oxidation Processes minimize bromate formation by generating hydroxyl radicals that degrade contaminants without promoting bromide oxidation.
Sequential Disinfection
Sequential disinfection combines chlorination and advanced oxidation processes (AOPs) to enhance water treatment by maximizing pathogen inactivation and minimizing disinfection byproducts; chlorination provides a persistent residual effect while AOPs generate hydroxyl radicals that degrade complex organic contaminants. Integrating these methods in sequence improves water safety, reduces toxic compounds, and meets stringent regulatory standards for potable water quality.
UV/Hydrogen Peroxide Synergy
Chlorination remains a widely used water disinfection method due to its effectiveness in eliminating pathogens and providing residual protection; however, the UV/Hydrogen Peroxide advanced oxidation process (AOP) offers superior degradation of micropollutants and emerging contaminants through hydroxyl radical generation. The synergy between UV radiation and hydrogen peroxide enhances hydroxyl radical production, enabling rapid oxidation of organic compounds while minimizing harmful disinfection byproducts typically associated with chlorination.
Targeted Micropollutant Removal
Chlorination effectively inactivates bacteria and viruses but often falls short in removing targeted micropollutants such as pharmaceuticals and endocrine disruptors. Advanced Oxidation Processes (AOPs) utilize highly reactive radicals like hydroxyl radicals to achieve superior degradation of these persistent contaminants, enhancing water treatment performance.
Ozone-Enhanced Chlorination
Ozone-enhanced chlorination combines the strong oxidizing power of ozone with chlorination to improve disinfection efficiency and reduce chlorinated byproduct formation in water treatment. This hybrid process effectively targets resistant pathogens and organic contaminants while minimizing the formation of harmful trihalomethanes (THMs) and haloacetic acids (HAAs).
Chlorination vs Advanced Oxidation Process Infographic
 industrydif.com
industrydif.com