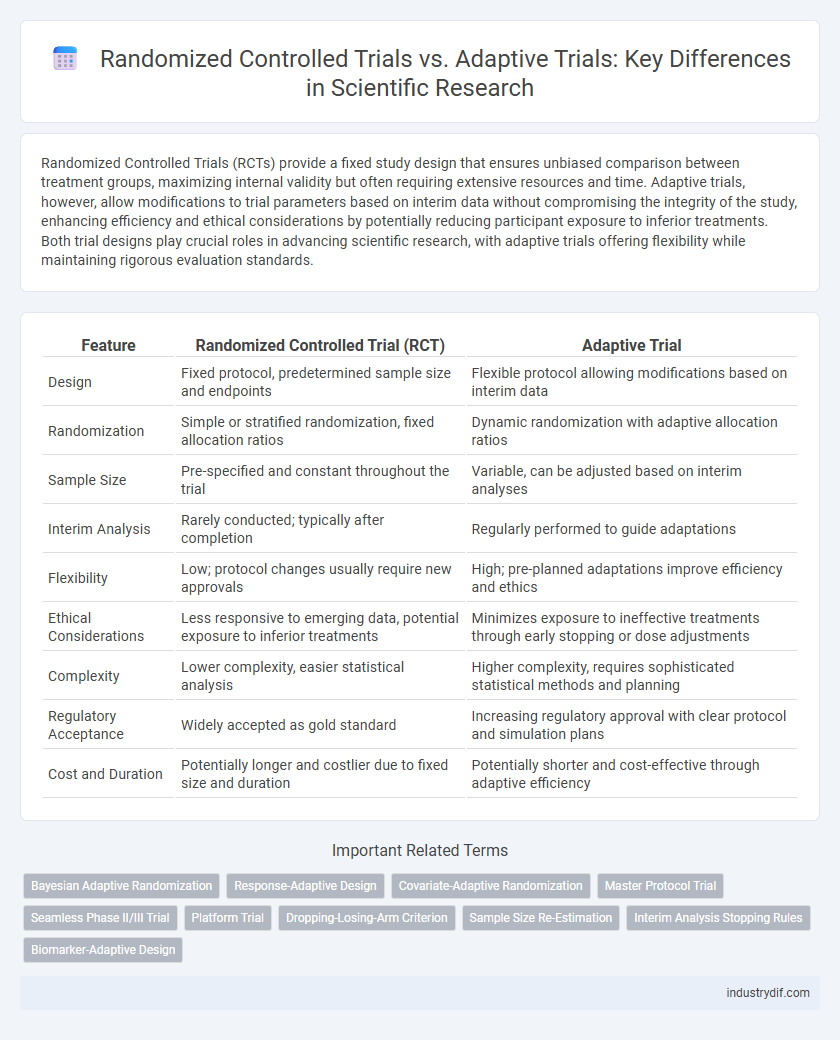
Randomized Controlled Trials (RCTs) provide a fixed study design that ensures unbiased comparison between treatment groups, maximizing internal validity but often requiring extensive resources and time. Adaptive trials, however, allow modifications to trial parameters based on interim data without compromising the integrity of the study, enhancing efficiency and ethical considerations by potentially reducing participant exposure to inferior treatments. Both trial designs play crucial roles in advancing scientific research, with adaptive trials offering flexibility while maintaining rigorous evaluation standards.
Table of Comparison
| Feature | Randomized Controlled Trial (RCT) | Adaptive Trial |
|---|---|---|
| Design | Fixed protocol, predetermined sample size and endpoints | Flexible protocol allowing modifications based on interim data |
| Randomization | Simple or stratified randomization, fixed allocation ratios | Dynamic randomization with adaptive allocation ratios |
| Sample Size | Pre-specified and constant throughout the trial | Variable, can be adjusted based on interim analyses |
| Interim Analysis | Rarely conducted; typically after completion | Regularly performed to guide adaptations |
| Flexibility | Low; protocol changes usually require new approvals | High; pre-planned adaptations improve efficiency and ethics |
| Ethical Considerations | Less responsive to emerging data, potential exposure to inferior treatments | Minimizes exposure to ineffective treatments through early stopping or dose adjustments |
| Complexity | Lower complexity, easier statistical analysis | Higher complexity, requires sophisticated statistical methods and planning |
| Regulatory Acceptance | Widely accepted as gold standard | Increasing regulatory approval with clear protocol and simulation plans |
| Cost and Duration | Potentially longer and costlier due to fixed size and duration | Potentially shorter and cost-effective through adaptive efficiency |
Definitions: Randomized Controlled Trials and Adaptive Trials
Randomized Controlled Trials (RCTs) are experimental studies where participants are randomly assigned to either the intervention group or the control group to evaluate the efficacy of medical treatments with high internal validity. Adaptive Trials utilize pre-planned modifications to trial parameters, such as sample size or treatment arms, based on interim data analysis without compromising the study's integrity or validity. Both methodologies aim to optimize clinical research, with RCTs emphasizing rigid protocol adherence and Adaptive Trials enhancing flexibility and efficiency in assessing therapeutic interventions.
Key Differences Between RCTs and Adaptive Trials
Randomized Controlled Trials (RCTs) employ fixed protocols with pre-specified sample sizes and endpoints, maintaining strict control over treatment allocation to minimize bias. Adaptive Trials incorporate real-time data analysis to modify trial parameters such as sample size, dosages, or patient selection criteria, enhancing flexibility and efficiency. The key differences lie in RCTs' rigidity and fixed design versus Adaptive Trials' dynamic adjustments based on interim results, leading to potentially faster decision-making and resource optimization.
Methodological Approaches: Fixed vs. Flexible Designs
Randomized Controlled Trials (RCTs) utilize a fixed design that maintains a predetermined sample size and statistical analysis plan throughout the study, ensuring methodological rigor and reducing bias. Adaptive Trials employ flexible designs that allow modifications to sample size, dosage, or patient allocation based on interim data, enhancing efficiency and ethical considerations. The choice between fixed RCTs and adaptive designs hinges on the study's objectives, with adaptive trials offering dynamic adjustments that can accelerate decision-making without compromising scientific validity.
Statistical Considerations in RCTs and Adaptive Trials
Randomized Controlled Trials (RCTs) implement fixed sample sizes and predefined statistical analysis plans to control type I error and maintain power, ensuring rigorous hypothesis testing. Adaptive trials incorporate interim analyses and allow modifications, such as sample size re-estimation or treatment arm adjustments, while using complex statistical methods like alpha spending functions to preserve error rates. Statistical considerations in adaptive trials require advanced modeling and simulation techniques to address potential biases and maintain validity in inference compared to traditional RCT designs.
Advantages and Limitations of Randomized Controlled Trials
Randomized Controlled Trials (RCTs) offer rigorous control over confounding variables, enabling high internal validity and minimizing bias through random allocation and blinding. However, RCTs often face limitations such as high costs, extended timelines, and reduced flexibility to adapt protocols based on interim results. Their stringent inclusion criteria can limit external validity, potentially affecting the generalizability of findings to broader patient populations.
Pros and Cons of Adaptive Trial Designs
Adaptive trial designs enhance efficiency by allowing modifications to sample size, treatment arms, or allocation ratios based on interim data, potentially reducing costs and patient exposure to ineffective treatments. They pose statistical and operational challenges, including increased complexity in trial planning, requirement for advanced statistical methods, and potential biases impacting validity. Regulatory acceptance is evolving, demanding robust justification and transparency to ensure data integrity and interpretability.
Regulatory Perspectives on RCTs and Adaptive Trials
Regulatory agencies traditionally favor randomized controlled trials (RCTs) due to their well-established methodology and clear control over Type I error rates, providing robust evidence for drug approval. Adaptive trials, while offering flexibility and efficiency by allowing modifications based on interim data, face regulatory scrutiny to ensure statistical validity and maintain trial integrity. Agencies such as the FDA and EMA provide guidance frameworks emphasizing pre-specified adaptation rules, stringent control of multiplicity, and comprehensive simulation studies to support adaptive trial designs.
Application Scenarios: When to Choose RCT vs. Adaptive Trial
Randomized Controlled Trials (RCTs) are preferred in scenarios requiring high internal validity and clear causal inference, especially in early-phase drug development or when regulatory approval demands rigorous evidence. Adaptive trials offer flexibility suitable for complex diseases or rapidly evolving conditions, allowing modifications based on interim data to optimize resource use and ethical considerations. Selecting between RCTs and adaptive trials depends on factors like the trial phase, disease complexity, urgency of results, and regulatory requirements.
Impact on Clinical Development Timelines
Randomized Controlled Trials (RCTs) provide rigorous, standardized data but often require longer durations to reach conclusive results, potentially extending clinical development timelines. Adaptive Trials optimize resource use through interim analyses, enabling modifications such as dosage adjustments or sample size re-estimation, which can shorten development phases. Implementing adaptive designs increases efficiency in decision-making, accelerating timelines without compromising the validity and reliability of clinical outcomes.
Future Trends in Trial Designs: Evolving Standards
Future trends in trial designs emphasize the increasing integration of adaptive trials, leveraging real-time data to optimize patient allocation and enhance trial efficiency compared to traditional randomized controlled trials (RCTs). Advances in machine learning and biomarker-driven stratification are driving more personalized interventions within adaptive frameworks. Regulatory agencies are progressively updating guidelines to support flexible protocols, enabling faster decision-making and improved resource utilization in clinical research.
Related Important Terms
Bayesian Adaptive Randomization
Bayesian adaptive randomization in clinical trials dynamically updates patient allocation probabilities based on accumulating efficacy and safety data, enhancing trial efficiency and ethical treatment distribution compared to traditional randomized controlled trials (RCTs). This approach leverages posterior probability models to favor more promising treatments, reducing exposure to inferior arms and accelerating decision-making in drug development.
Response-Adaptive Design
Response-adaptive design in randomized controlled trials (RCTs) enables dynamic modification of patient allocation ratios based on interim outcome data, optimizing treatment efficacy and ethical considerations by favoring better-performing interventions. This adaptive approach contrasts with traditional fixed RCT designs by enhancing statistical power and reducing resource use without compromising trial integrity.
Covariate-Adaptive Randomization
Covariate-adaptive randomization in randomized controlled trials (RCTs) ensures balanced distribution of prognostic factors across treatment groups, reducing bias and improving statistical efficiency. Adaptive trials utilize this method to dynamically adjust participant allocation based on covariate profiles, enhancing trial flexibility and precision in estimating treatment effects.
Master Protocol Trial
Master protocol trials integrate multiple sub-studies under a single overarching framework, enhancing efficiency by concurrently evaluating various interventions or patient subgroups compared to traditional randomized controlled trials (RCTs) that test a single hypothesis. Adaptive trial designs within master protocols allow pre-specified modifications based on interim results, optimizing resource use and accelerating decision-making without compromising statistical integrity.
Seamless Phase II/III Trial
Seamless Phase II/III trials integrate the objectives of traditional randomized controlled trials (RCTs) by combining exploratory and confirmatory phases, thereby optimizing resource allocation and speeding up drug development. Adaptive trial designs enable pre-planned modifications based on interim data without compromising the trial's integrity, enhancing flexibility and efficiency compared to fixed-design RCTs.
Platform Trial
Platform trials, a subtype of adaptive trials, allow simultaneous evaluation of multiple treatments within a single protocol using a common control group, enhancing efficiency and flexibility in clinical research. Randomized Controlled Trials (RCTs) traditionally test a fixed number of interventions with rigid protocols, whereas platform trials dynamically modify treatments and patient allocations based on interim analyses to accelerate decision-making and resource utilization.
Dropping-Losing-Arm Criterion
The Dropping-Losing-Arm Criterion in randomized controlled trials (RCTs) involves eliminating treatment arms that demonstrate inferior efficacy or safety based on predefined statistical thresholds, thus preserving trial integrity and resource allocation. Adaptive trials enhance this process by continuously analyzing interim data to dynamically drop underperforming arms, increasing trial efficiency and ethical responsiveness without compromising statistical rigor.
Sample Size Re-Estimation
Randomized Controlled Trials (RCTs) typically require fixed sample sizes determined before the study begins, while Adaptive Trials employ Sample Size Re-Estimation techniques to adjust participant numbers based on interim data, enhancing statistical power and resource efficiency. This adaptive approach allows for dynamic modifications that address uncertainties in effect size or variance, optimizing trial outcomes without compromising validity.
Interim Analysis Stopping Rules
In randomized controlled trials (RCTs), interim analysis stopping rules are typically predefined and rigid, allowing trial termination only upon meeting strict efficacy or safety thresholds to maintain statistical integrity. Adaptive trials employ flexible interim analyses that enable dynamic modification of trial parameters, such as sample size or treatment arms, based on accumulating data while controlling type I error rates through complex statistical methods.
Biomarker-Adaptive Design
Biomarker-adaptive design in clinical trials integrates real-time biomarker data to modify trial parameters, enhancing patient stratification and treatment efficacy compared to traditional randomized controlled trials (RCTs). This approach allows dynamic adjustments in sample size, treatment arms, or eligibility criteria based on interim analyses, improving the precision and personalization of therapeutic interventions.
Randomized Controlled Trial vs Adaptive Trial Infographic
 industrydif.com
industrydif.com