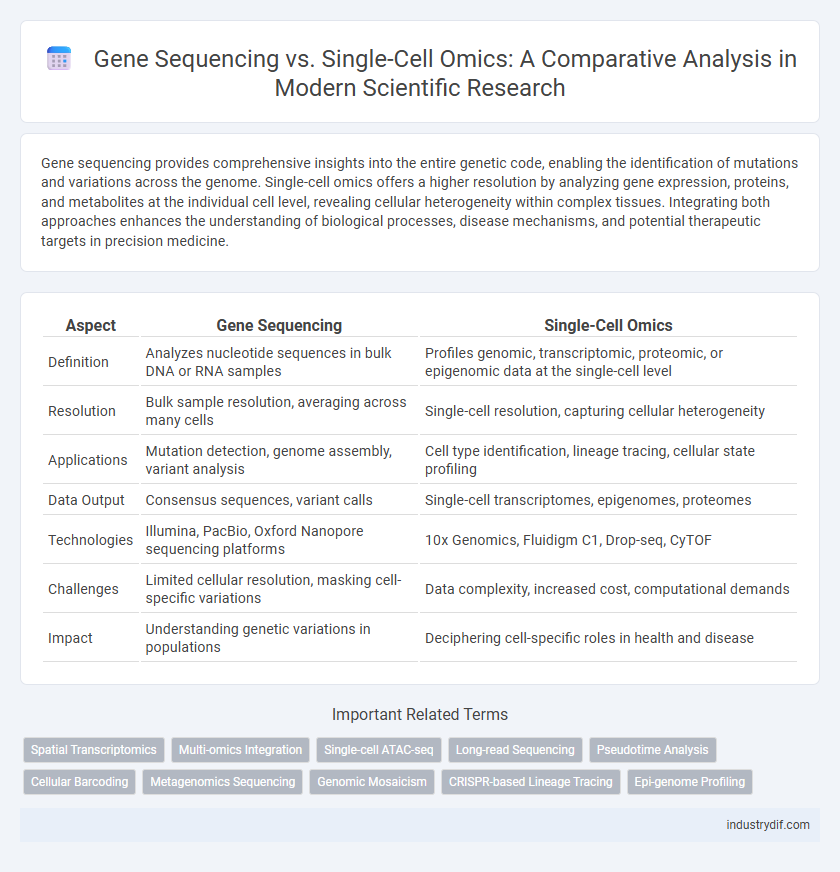
Gene sequencing provides comprehensive insights into the entire genetic code, enabling the identification of mutations and variations across the genome. Single-cell omics offers a higher resolution by analyzing gene expression, proteins, and metabolites at the individual cell level, revealing cellular heterogeneity within complex tissues. Integrating both approaches enhances the understanding of biological processes, disease mechanisms, and potential therapeutic targets in precision medicine.
Table of Comparison
| Aspect | Gene Sequencing | Single-Cell Omics |
|---|---|---|
| Definition | Analyzes nucleotide sequences in bulk DNA or RNA samples | Profiles genomic, transcriptomic, proteomic, or epigenomic data at the single-cell level |
| Resolution | Bulk sample resolution, averaging across many cells | Single-cell resolution, capturing cellular heterogeneity |
| Applications | Mutation detection, genome assembly, variant analysis | Cell type identification, lineage tracing, cellular state profiling |
| Data Output | Consensus sequences, variant calls | Single-cell transcriptomes, epigenomes, proteomes |
| Technologies | Illumina, PacBio, Oxford Nanopore sequencing platforms | 10x Genomics, Fluidigm C1, Drop-seq, CyTOF |
| Challenges | Limited cellular resolution, masking cell-specific variations | Data complexity, increased cost, computational demands |
| Impact | Understanding genetic variations in populations | Deciphering cell-specific roles in health and disease |
Introduction to Gene Sequencing and Single-Cell Omics
Gene sequencing involves determining the precise order of nucleotides within a DNA molecule, enabling insights into genetic variations and functions at the molecular level. Single-cell omics expands this by analyzing genomic, transcriptomic, proteomic, and epigenomic data from individual cells, providing high-resolution understanding of cellular heterogeneity and complex biological systems. These technologies complement each other by combining bulk genetic information with single-cell specificity, advancing personalized medicine and biological research.
Fundamentals of Gene Sequencing Technologies
Gene sequencing technologies, including Sanger sequencing and next-generation sequencing (NGS), provide comprehensive analysis of nucleotide sequences, enabling detailed insights into genetic mutations and variations. Fundamental principles involve DNA extraction, library preparation, amplification, and high-throughput reading, contrasting with single-cell omics that emphasize cellular heterogeneity by analyzing genomic, transcriptomic, or proteomic data at the individual cell level. Innovations in sequencing platforms have significantly improved accuracy, speed, and scalability, driving advancements in precision medicine and functional genomics.
Overview of Single-Cell Omics Approaches
Single-cell omics technologies enable the profiling of genomic, transcriptomic, proteomic, and epigenomic information at the resolution of individual cells, offering unprecedented insights into cellular heterogeneity. Techniques such as single-cell RNA sequencing (scRNA-seq), single-cell ATAC-seq, and spatial transcriptomics provide detailed molecular characterization that surpasses bulk gene sequencing approaches by capturing cell-specific variations and states. These methods are crucial for understanding complex biological processes, disease mechanisms, and developing precision medicine strategies.
Key Differences: Bulk Sequencing vs. Single-Cell Resolution
Gene sequencing typically analyzes bulk samples, providing averaged genetic information from thousands to millions of cells, which can mask cellular heterogeneity. Single-cell omics enables the examination of individual cells, revealing distinct genetic, transcriptomic, and epigenomic profiles critical for understanding cellular diversity and complex biological processes. This high-resolution approach uncovers rare cell populations and dynamic cellular states that bulk sequencing cannot detect.
Data Output and Analytical Complexity
Gene sequencing generates large-scale nucleotide data that provide comprehensive genomic information but often lack resolution at the individual cell level. Single-cell omics produces multidimensional datasets encompassing transcriptomics, proteomics, and epigenomics, offering high-resolution insights into cellular heterogeneity and dynamic biological processes. The analytical complexity of single-cell omics surpasses traditional gene sequencing due to the need for sophisticated computational tools to manage sparse, high-dimensional data and to identify meaningful patterns across diverse cell populations.
Applications in Genomic Research and Medicine
Gene sequencing enables comprehensive analysis of entire genomes, facilitating identification of genetic mutations associated with diseases and personalized medicine development. Single-cell omics provides high-resolution insights into cellular heterogeneity by profiling genomics, transcriptomics, and proteomics at the individual cell level, crucial for understanding complex tissue microenvironments and tumor evolution. Integration of both technologies accelerates discoveries in cancer biology, immunology, and rare genetic disorders, driving precision diagnostics and targeted therapies.
Advantages and Limitations of Gene Sequencing
Gene sequencing provides comprehensive insights into the entire genome, enabling the identification of genetic variants and mutations with high accuracy, which is essential for understanding hereditary diseases and cancer genomics. Its limitations include the inability to capture dynamic cellular heterogeneity and temporal gene expression changes that single-cell omics can resolve by analyzing individual cells. Despite lower resolution at the cellular level, gene sequencing remains a cost-effective and scalable method for large population studies and variant discovery.
Unique Insights Offered by Single-Cell Omics
Single-cell omics provides unique insights by analyzing gene expression, protein levels, and metabolic states at the resolution of individual cells, revealing cellular heterogeneity hidden in bulk gene sequencing. This approach enables the identification of rare cell populations and elucidates complex cellular interactions within tissues. By capturing dynamic cellular states, single-cell omics advances our understanding of development, disease progression, and therapeutic responses.
Integrative Multi-Omics: Future Directions
Integrative multi-omics combines gene sequencing data with single-cell omics to unravel complex biological systems at unprecedented resolution. This approach leverages genomic, transcriptomic, and proteomic data from individual cells to identify novel biomarkers and therapeutic targets. Future directions emphasize scalable computational tools and AI-driven analytics to enhance data integration and precision medicine applications.
Conclusion: Choosing the Right Approach for Scientific Inquiry
Selecting the appropriate method between gene sequencing and single-cell omics depends on the specific research objectives, with gene sequencing providing comprehensive genetic information across populations while single-cell omics offers detailed molecular insights at individual cell resolution. Gene sequencing excels in detecting genetic variants and mutations in bulk samples, essential for population genetics and disease association studies. Single-cell omics is indispensable for understanding cellular heterogeneity, transcriptional profiles, and dynamic biological processes, making it the preferred choice for studies focused on cellular diversity and functional genomics.
Related Important Terms
Spatial Transcriptomics
Spatial transcriptomics integrates gene sequencing data with spatial information, enabling precise mapping of gene expression within tissue architecture at single-cell resolution. This advanced approach enhances insights into cellular heterogeneity and tissue microenvironment interactions, surpassing traditional single-cell omics by preserving spatial context during transcriptomic analysis.
Multi-omics Integration
Gene sequencing provides comprehensive information on genomic variations, while single-cell omics offers high-resolution insights into cellular heterogeneity by analyzing transcriptomic, proteomic, and epigenomic profiles at the individual cell level. Multi-omics integration leverages these complementary datasets to enhance the understanding of complex biological systems and disease mechanisms, enabling precise biomarker identification and therapeutic target discovery.
Single-cell ATAC-seq
Single-cell ATAC-seq enables the profiling of chromatin accessibility at the individual cell level, offering insights into gene regulatory mechanisms that traditional bulk gene sequencing cannot resolve. This technique uncovers cellular heterogeneity and epigenetic landscapes with high resolution, advancing our understanding of gene expression regulation in complex tissues.
Long-read Sequencing
Long-read sequencing technologies, such as PacBio and Oxford Nanopore, provide comprehensive genomic information by accurately capturing structural variants and complex regions that are often missed by short-read sequencing in single-cell omics. These advances enable deeper insights into cellular heterogeneity, epigenetic modifications, and transcript isoforms, enhancing the resolution of gene expression and functional genomics studies at the single-cell level.
Pseudotime Analysis
Pseudotime analysis in gene sequencing orders cells along a trajectory reflecting dynamic biological processes, enabling reconstruction of temporal changes from static single-cell snapshots. Single-cell omics integrate multi-dimensional data such as transcriptomics and epigenomics, enhancing resolution and accuracy of pseudotime inference to reveal cellular differentiation pathways and lineage relationships.
Cellular Barcoding
Cellular barcoding in gene sequencing enables precise tracking of individual cell lineages by assigning unique DNA tags, facilitating high-resolution analysis of cellular heterogeneity. In single-cell omics, this technique integrates multi-omics data from the same cell, enhancing insights into gene expression, epigenetics, and protein interactions at a single-cell level.
Metagenomics Sequencing
Metagenomics sequencing enables comprehensive analysis of microbial communities by decoding collective genomes from environmental samples, surpassing traditional gene sequencing which targets individual genes or organisms. Single-cell omics offers detailed profiles of cellular heterogeneity but metagenomics provides broader insights into microbial diversity and ecosystem functions, critical for applications in microbial ecology and diagnostics.
Genomic Mosaicism
Gene sequencing provides a comprehensive analysis of DNA variations across bulk cell populations, while single-cell omics enables high-resolution profiling of genetic and epigenetic heterogeneity within individual cells. This distinction is critical for studying genomic mosaicism, as single-cell omics reveals cell-specific mutations and structural variations that bulk sequencing methods may obscure.
CRISPR-based Lineage Tracing
CRISPR-based lineage tracing enhances gene sequencing and single-cell omics by enabling precise mapping of cellular ancestries and dynamic gene expression changes at a single-cell resolution. This technology leverages inducible CRISPR-Cas9 systems to introduce unique genetic barcodes, facilitating high-throughput tracking of cell fate and lineage relationships in complex tissues.
Epi-genome Profiling
Gene sequencing provides a comprehensive analysis of DNA sequences, while single-cell omics enables high-resolution profiling of epigenomic modifications within individual cells, revealing heterogeneity in gene regulation. Epigenome profiling through techniques like single-cell ATAC-seq uncovers chromatin accessibility patterns, crucial for understanding cellular differentiation and disease mechanisms at the single-cell level.
Gene Sequencing vs Single-Cell Omics Infographic
 industrydif.com
industrydif.com