Animal testing has long been a cornerstone in scientific research for understanding disease mechanisms and evaluating drug safety, but ethical concerns and species differences challenge its applicability. Organotypic culture models replicate the three-dimensional architecture and cellular complexity of tissues, offering more physiologically relevant data while reducing reliance on live animals. These in vitro systems enhance insight into tissue-specific responses and accelerate drug development with improved predictive accuracy.
Table of Comparison
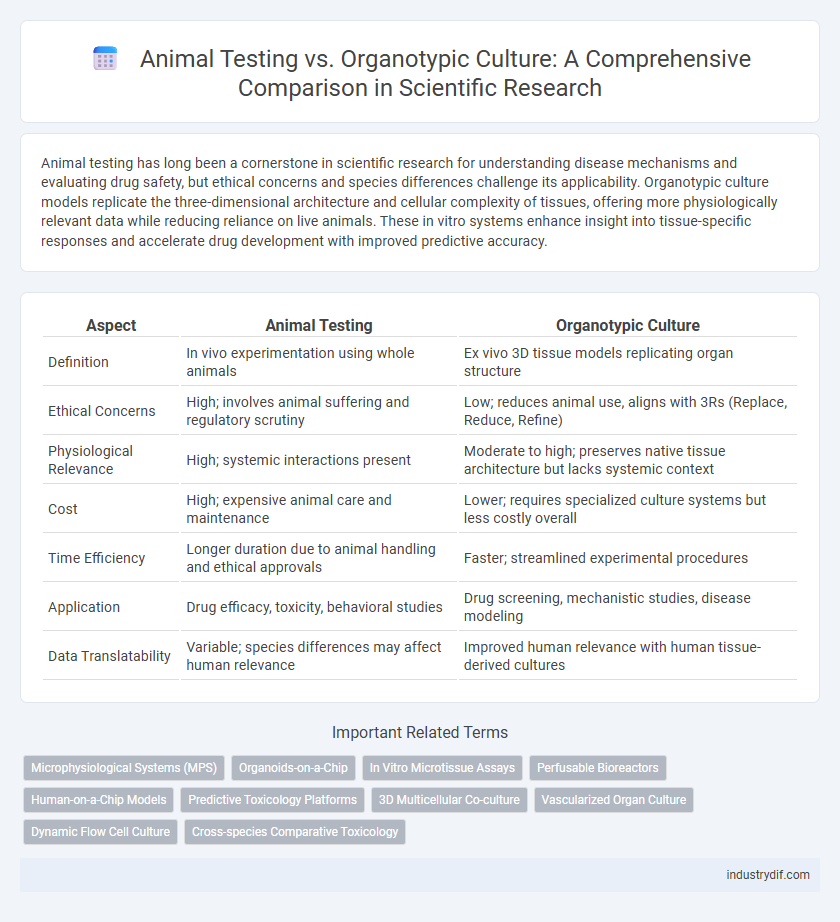
| Aspect | Animal Testing | Organotypic Culture |
|---|---|---|
| Definition | In vivo experimentation using whole animals | Ex vivo 3D tissue models replicating organ structure |
| Ethical Concerns | High; involves animal suffering and regulatory scrutiny | Low; reduces animal use, aligns with 3Rs (Replace, Reduce, Refine) |
| Physiological Relevance | High; systemic interactions present | Moderate to high; preserves native tissue architecture but lacks systemic context |
| Cost | High; expensive animal care and maintenance | Lower; requires specialized culture systems but less costly overall |
| Time Efficiency | Longer duration due to animal handling and ethical approvals | Faster; streamlined experimental procedures |
| Application | Drug efficacy, toxicity, behavioral studies | Drug screening, mechanistic studies, disease modeling |
| Data Translatability | Variable; species differences may affect human relevance | Improved human relevance with human tissue-derived cultures |
Introduction to Animal Testing and Organotypic Culture
Animal testing has historically been the primary method for evaluating drug safety and efficacy, involving live animals to study physiological responses and toxicological effects. Organotypic culture represents an advanced in vitro technique that simulates the three-dimensional architecture and cellular complexity of native tissues, enabling more accurate modeling of human biology. Both methods provide critical insights but differ significantly in ethical considerations, cost, and translational relevance to human health outcomes.
Historical Overview of In Vivo and Ex Vivo Methods
Animal testing has long served as a cornerstone in biomedical research, providing critical insights into disease mechanisms and drug safety since the early 20th century. Organotypic culture techniques emerged in the late 20th century as innovative ex vivo methods that replicate the three-dimensional architecture and cellular complexity of tissues. These advancements have progressively shifted research paradigms toward more ethical and physiologically relevant models, reducing reliance on traditional in vivo experiments.
Scientific Principles Behind Animal Testing
Animal testing relies on biological models to study complex physiological systems and predict human responses, emphasizing principles such as reproducibility, dose-response relationships, and ethical considerations guided by the 3Rs (Replacement, Reduction, Refinement). It enables researchers to observe systemic interactions and potential side effects in a whole organism, which organotypic cultures, despite their advanced cellular architecture, cannot fully replicate. Regulatory frameworks often mandate animal testing to ensure safety and efficacy before clinical trials, underscoring its foundational role in biomedical research.
Fundamentals of Organotypic Culture Systems
Organotypic culture systems replicate the 3D architecture and cellular diversity of native tissues, enabling more accurate modeling of in vivo conditions compared to traditional animal testing. These cultures maintain tissue-specific extracellular matrix components and cell-cell interactions essential for studying physiological and pathological processes. By preserving native tissue complexity, organotypic cultures provide a scalable, ethical alternative for drug screening and toxicity assessment.
Comparative Analysis: Biological Relevance and Predictive Value
Animal testing provides whole-organism context, allowing assessment of systemic interactions and complex physiological responses, but often suffers from species-specific differences that limit direct human relevance. Organotypic culture models, derived from human tissues, offer improved biological relevance by maintaining native cellular architecture and microenvironment, enhancing the predictive value for human-specific outcomes. Comparative analyses demonstrate organotypic cultures reduce ethical concerns and better predict clinical efficacy and toxicity, although full integration with in vivo data remains essential for comprehensive biomedical research.
Ethical Considerations in Preclinical Research
Animal testing raises ethical concerns due to animal welfare and the potential for pain and distress, which has led to increasing support for alternative methods like organotypic culture that better mimic human tissue responses. Organotypic cultures reduce the use of live animals by providing three-dimensional, physiologically relevant models that improve the predictability of preclinical research outcomes while adhering to the 3Rs principle of Replacement, Reduction, and Refinement. These ethical advantages promote more humane and scientifically reliable approaches in drug development and toxicology testing.
Regulatory Requirements and Guidelines
Regulatory requirements for animal testing are governed by agencies such as the FDA, EMA, and OECD, mandating in vivo data for toxicity, safety, and efficacy assessments before clinical trials. Organotypic culture models, including 3D tissue cultures and microphysiological systems, are increasingly integrated into guidelines for reducing animal use while providing human-relevant data under frameworks like the 3Rs principle (Replacement, Reduction, Refinement). Compliance with ICH M3(R2) and REACH regulations supports validation of organotypic cultures as alternative methods to fulfill safety testing requirements in pharmaceutical and chemical development.
Advancements and Limitations of Organotypic Cultures
Organotypic cultures provide a three-dimensional cellular environment that closely mimics in vivo tissue architecture, enabling more accurate modeling of physiological and pathological conditions compared to traditional two-dimensional animal testing. Recent advancements in bioengineering and microfluidics have enhanced the complexity and viability of organotypic cultures, facilitating high-throughput drug screening and toxicity assessment with reduced ethical concerns. However, limitations include challenges in replicating systemic interactions and immune responses, which remain critical for comprehensive biological studies and translational research.
Applications in Drug Discovery and Toxicology
Organotypic cultures provide a three-dimensional, physiologically relevant environment that closely mimics in vivo tissue architecture, enhancing predictive accuracy in drug discovery and toxicology compared to traditional animal testing. These cultures enable high-throughput screening of drug candidates and mechanistic studies of toxicity pathways while reducing ethical concerns linked to animal use. Integrating organotypic models with advanced imaging and molecular techniques accelerates identification of drug efficacy and safety profiles, supporting more reliable translational research.
Future Perspectives in Scientific Testing Methods
Advancements in organotypic culture models have shown significant promise for reducing reliance on animal testing by replicating human tissue architecture and function more accurately. Emerging techniques such as 3D bioprinting and microfluidic platforms are enhancing the physiological relevance and throughput of these in vitro systems. Future scientific testing methods will increasingly integrate organotypic cultures with computational modeling to improve predictive accuracy and ethical standards in biomedical research.
Related Important Terms
Microphysiological Systems (MPS)
Microphysiological Systems (MPS) provide a cutting-edge alternative to traditional animal testing by replicating human organ functions through organotypic culture techniques, enhancing the predictive accuracy of drug responses and reducing ethical concerns. These systems integrate multiple cell types in a microengineered environment to simulate in vivo physiological conditions, offering a scalable and reproducible platform for toxicology and disease modeling.
Organoids-on-a-Chip
Organoids-on-a-chip represent a cutting-edge alternative to traditional animal testing by replicating human tissue architecture and physiological responses within microengineered environments, enhancing predictive accuracy for drug toxicity and disease modeling. This technology integrates organoids with microfluidic systems to mimic organ-level functions, reducing reliance on animal models and improving translational relevance in biomedical research.
In Vitro Microtissue Assays
In vitro microtissue assays offer a promising alternative to animal testing by replicating human tissue architecture and cellular interactions, enabling more accurate toxicity and efficacy assessments. These organotypic cultures improve translational relevance and reduce ethical concerns associated with animal models in biomedical research.
Perfusable Bioreactors
Perfusable bioreactors in organotypic culture systems replicate in vivo microenvironments by enabling dynamic nutrient and oxygen exchange, significantly enhancing tissue viability and functionality compared to static animal testing models. This advanced in vitro approach reduces ethical concerns and improves translational relevance for drug screening and toxicological assessments.
Human-on-a-Chip Models
Human-on-a-chip models offer advanced organotypic culture systems that replicate human physiological responses more accurately than traditional animal testing, reducing ethical concerns and enhancing predictive validity in drug development. These microfluidic platforms integrate multiple tissue types to simulate complex organ interactions, providing scalable, high-throughput testing environments for toxicology and disease modeling.
Predictive Toxicology Platforms
Organotypic culture systems offer more human-relevant predictive toxicology platforms by replicating tissue architecture and cellular interactions, reducing the species-specific discrepancies inherent in animal testing. Advances in 3D cell culture and microfluidic technologies enhance the accuracy of toxicity assessments while minimizing ethical concerns associated with traditional animal models.
3D Multicellular Co-culture
3D multicellular co-culture models in organotypic culture replicate complex tissue architecture and cellular interactions more accurately than traditional animal testing, enhancing the predictive validity of toxicological and pharmacological studies. These models enable high-throughput screening while reducing ethical concerns and variability associated with in vivo experiments, offering a scalable platform for personalized medicine and disease modeling.
Vascularized Organ Culture
Vascularized organotypic cultures replicate native tissue microenvironments by integrating functional blood vessels, providing more physiologically relevant models than traditional animal testing. These advanced cultures enable precise studies of vascular interactions, drug responses, and disease mechanisms while reducing ethical concerns and interspecies variability inherent in animal models.
Dynamic Flow Cell Culture
Dynamic flow cell culture in organotypic models replicates physiological conditions more accurately than static animal testing environments, enhancing cellular responses and tissue functionality. This approach reduces reliance on animal testing by providing controlled fluid shear stress and nutrient exchange, improving drug metabolism and toxicity assessments.
Cross-species Comparative Toxicology
Cross-species comparative toxicology leverages organotypic culture models to replicate human tissue responses more accurately than traditional animal testing, enhancing predictive validity for toxicity assessment. This approach reduces interspecies variability inherent in animal models by using human-derived 3D tissue systems, facilitating mechanistic insights and improving the translational relevance of toxicological data.
Animal Testing vs Organotypic Culture Infographic
 industrydif.com
industrydif.com