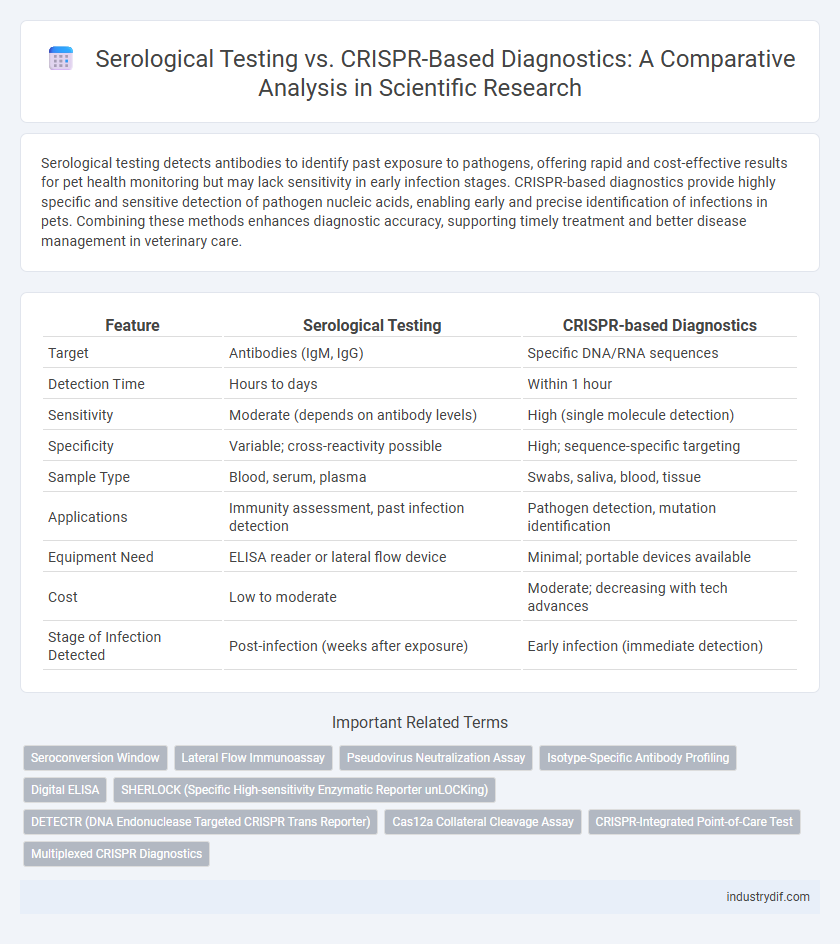
Serological testing detects antibodies to identify past exposure to pathogens, offering rapid and cost-effective results for pet health monitoring but may lack sensitivity in early infection stages. CRISPR-based diagnostics provide highly specific and sensitive detection of pathogen nucleic acids, enabling early and precise identification of infections in pets. Combining these methods enhances diagnostic accuracy, supporting timely treatment and better disease management in veterinary care.
Table of Comparison
| Feature | Serological Testing | CRISPR-based Diagnostics |
|---|---|---|
| Target | Antibodies (IgM, IgG) | Specific DNA/RNA sequences |
| Detection Time | Hours to days | Within 1 hour |
| Sensitivity | Moderate (depends on antibody levels) | High (single molecule detection) |
| Specificity | Variable; cross-reactivity possible | High; sequence-specific targeting |
| Sample Type | Blood, serum, plasma | Swabs, saliva, blood, tissue |
| Applications | Immunity assessment, past infection detection | Pathogen detection, mutation identification |
| Equipment Need | ELISA reader or lateral flow device | Minimal; portable devices available |
| Cost | Low to moderate | Moderate; decreasing with tech advances |
| Stage of Infection Detected | Post-infection (weeks after exposure) | Early infection (immediate detection) |
Overview of Serological Testing in Scientific Diagnostics
Serological testing detects antibodies in blood, indicating past exposure to pathogens such as viruses or bacteria, and plays a critical role in epidemiological surveillance and immune response assessment. It utilizes techniques like enzyme-linked immunosorbent assays (ELISA) and lateral flow assays to measure immunoglobulins IgG, IgM, and IgA, providing valuable data on infection history and vaccine efficacy. This approach contrasts with CRISPR-based diagnostics that identify active infections by detecting specific nucleic acid sequences with high sensitivity and rapid turnaround times.
Introduction to CRISPR-based Diagnostic Technologies
CRISPR-based diagnostic technologies utilize the precise gene-editing capabilities of CRISPR-Cas systems to detect nucleic acid sequences with high specificity and sensitivity, enabling rapid identification of pathogens or genetic mutations. Unlike traditional serological testing that detects antibodies or antigens, CRISPR diagnostics directly target and cleave specific DNA or RNA sequences, producing a detectable signal. This innovative approach offers potential for point-of-care testing with faster turnaround times and reduced false positives compared to conventional serological assays.
Mechanisms: How Serological Tests Work
Serological tests detect antibodies in blood, indicating past or present infection by recognizing specific immune proteins produced in response to a pathogen. These tests use antigens to bind with antibodies, forming complexes that produce measurable signals through methods like enzyme-linked immunosorbent assay (ELISA) or lateral flow assays. The sensitivity and specificity of serological testing depend on the targeted antibody isotypes (IgM, IgG) and the timing of sample collection relative to infection onset.
Mechanisms: CRISPR-based Detection Principles
CRISPR-based diagnostics utilize the Cas enzymes, such as Cas12 and Cas13, which bind to specific nucleic acid sequences and trigger collateral cleavage activity, enabling highly sensitive detection of target DNA or RNA. Upon target recognition, the activated Cas enzyme cleaves reporter molecules, producing fluorescent or colorimetric signals that indicate the presence of the pathogen. This mechanism provides rapid, precise, and amplification-free detection compared to traditional serological assays that measure antibodies or antigens indirectly.
Sensitivity and Specificity: Comparative Performance
Serological testing primarily detects antibodies and often shows lower sensitivity in early infection stages compared to CRISPR-based diagnostics, which directly identify viral nucleic acids with higher precision. CRISPR diagnostics demonstrate superior specificity through programmable guide RNAs that minimize false positives, while serological assays may cross-react with antibodies from similar pathogens. Studies reveal CRISPR methods achieving sensitivity rates upwards of 95% and specificity near 99%, outperforming many traditional serological tests in clinical accuracy.
Turnaround Time: Speed of Detection in Both Methods
Serological testing typically requires several hours to days due to antibody maturation and sample processing times, limiting rapid diagnosis. CRISPR-based diagnostics leverage gene-editing mechanisms to detect nucleic acids within 30 minutes to an hour, offering significantly faster turnaround times. This rapid detection capability enhances timely clinical decision-making and outbreak control efforts.
Scalability and Throughput in Laboratory Settings
Serological testing offers limited scalability due to dependence on antibody development timelines and potential cross-reactivity, which affects throughput in high-demand lab environments. CRISPR-based diagnostics enable rapid, sensitive detection with modular scalability, supporting multiplexed assays that significantly enhance throughput capacity. Integration of CRISPR platforms with automated workflows further streamlines processing, making them superior for large-scale diagnostic applications in clinical and research laboratories.
Cost and Resource Requirements
Serological testing offers a cost-effective and rapid approach for detecting antibodies with minimal laboratory infrastructure, making it accessible for large-scale screenings in resource-limited settings. CRISPR-based diagnostics require more specialized reagents and equipment, including Cas enzymes and guide RNAs, which increase overall expenses and necessitate skilled personnel for assay execution. Although CRISPR diagnostics provide higher specificity and sensitivity, their current resource demands limit widespread implementation compared to the more affordable serological methods.
Regulatory Approvals and Standardization
Regulatory approvals for serological testing are well-established, with many kits receiving FDA Emergency Use Authorization (EUA) and CE marking, facilitating widespread clinical adoption and standardization. CRISPR-based diagnostics, despite their rapid development and high sensitivity, face ongoing challenges in obtaining regulatory clearance due to novel assay formats and limited large-scale validation data. Standardization efforts for CRISPR diagnostics are emerging through international consortia aiming to create uniform protocols, whereas serological tests benefit from decades of assay optimization and harmonization guidelines.
Future Directions in Molecular Diagnostics
Serological testing remains crucial for identifying past infections by detecting antibodies, but CRISPR-based diagnostics offer unparalleled precision and speed in detecting nucleic acids, enabling real-time pathogen identification. Future directions in molecular diagnostics emphasize integrating CRISPR technology with point-of-care platforms to achieve rapid, cost-effective, and highly sensitive detection of emerging infectious diseases. Ongoing research aims to expand multiplexing capabilities, improve CRISPR assay robustness, and facilitate decentralized testing in diverse healthcare settings.
Related Important Terms
Seroconversion Window
Serological testing detects antibodies indicating past infection but is limited by the seroconversion window, typically 7-14 days post-infection, which delays diagnosis. CRISPR-based diagnostics identify viral RNA directly, enabling earlier detection during the acute phase before antibody production begins.
Lateral Flow Immunoassay
Lateral flow immunoassay (LFA) in serological testing offers rapid, point-of-care detection of antibodies, enabling effective monitoring of immune responses against infectious agents. CRISPR-based diagnostics provide higher sensitivity and specificity by targeting nucleic acid sequences, yet LFAs remain preferable for their simplicity, cost-effectiveness, and widespread applicability in decentralized testing environments.
Pseudovirus Neutralization Assay
Serological testing detects antibodies indicative of prior infection or vaccination, while CRISPR-based diagnostics offer rapid, highly specific nucleic acid detection for active infections. The Pseudovirus Neutralization Assay measures the functional capacity of antibodies to inhibit viral entry, providing critical data to correlate serological antibody levels with neutralizing activity against specific viral variants.
Isotype-Specific Antibody Profiling
Serological testing enables isotype-specific antibody profiling by detecting distinct immunoglobulin classes such as IgM, IgG, and IgA, providing insights into infection timeline and immune response durability. CRISPR-based diagnostics offer high sensitivity and specificity in nucleic acid detection but lack direct quantification of antibody isotypes, limiting their utility in detailed seroprofiling.
Digital ELISA
Digital ELISA enhances serological testing sensitivity by enabling single-molecule protein detection, outperforming conventional CRISPR-based diagnostics that primarily target nucleic acids. This technology offers precise quantification of low-abundance biomarkers, crucial for early disease diagnosis and monitoring immune responses.
SHERLOCK (Specific High-sensitivity Enzymatic Reporter unLOCKing)
SHERLOCK, a CRISPR-based diagnostic platform, leverages Cas13 enzymes to detect nucleic acids with ultra-high sensitivity and specificity, outperforming traditional serological tests that primarily identify antibodies and may miss early infections. This molecular assay enables rapid, point-of-care detection of viral RNA, offering crucial advantages for early diagnosis, outbreak control, and precision medicine compared to slower serological methods reliant on immune response timing.
DETECTR (DNA Endonuclease Targeted CRISPR Trans Reporter)
DETECTR (DNA Endonuclease Targeted CRISPR Trans Reporter) offers precise CRISPR-based diagnostics by combining Cas12 enzyme activity with fluorescent reporters to rapidly identify viral DNA sequences with high sensitivity and specificity. Unlike conventional serological testing that detects host antibodies and may yield delayed responses, DETECTR directly targets pathogen nucleic acids, enabling early infection detection crucial for timely clinical intervention.
Cas12a Collateral Cleavage Assay
The Cas12a collateral cleavage assay, a CRISPR-based diagnostic method, offers high sensitivity and specificity by detecting pathogen DNA through targeted cleavage and subsequent fluorescence signal amplification. Serological testing, while effective for identifying past infections via antibody detection, lacks the real-time pathogen detection capability and molecular precision demonstrated by Cas12a assays in early-stage diagnostics.
CRISPR-Integrated Point-of-Care Test
CRISPR-integrated point-of-care tests offer rapid, highly sensitive, and specific detection of nucleic acids, surpassing traditional serological testing that primarily detects antibodies and may exhibit delayed response post-infection. These CRISPR-based diagnostics enable real-time, on-site identification of pathogens and genetic markers, critical for timely clinical decision-making and infectious disease management.
Multiplexed CRISPR Diagnostics
Multiplexed CRISPR diagnostics enable simultaneous detection of multiple biomarkers with high sensitivity and specificity, outperforming traditional serological testing that typically identifies single antibodies or antigens. By leveraging CRISPR-Cas systems' precision and rapid readout capabilities, multiplexed assays facilitate comprehensive pathogen profiling and host immune response analysis in a single test.
Serological Testing vs CRISPR-based Diagnostics Infographic
 industrydif.com
industrydif.com