Hard water contains high levels of calcium and magnesium ions, which are replaced by sodium or potassium ions during the water softening process through cation exchange. This ion exchange reduces mineral buildup in pipes and appliances, preventing scale formation and improving water quality. Soft water enhances soap efficiency and leaves no residue, making it preferable for household and industrial use.
Table of Comparison
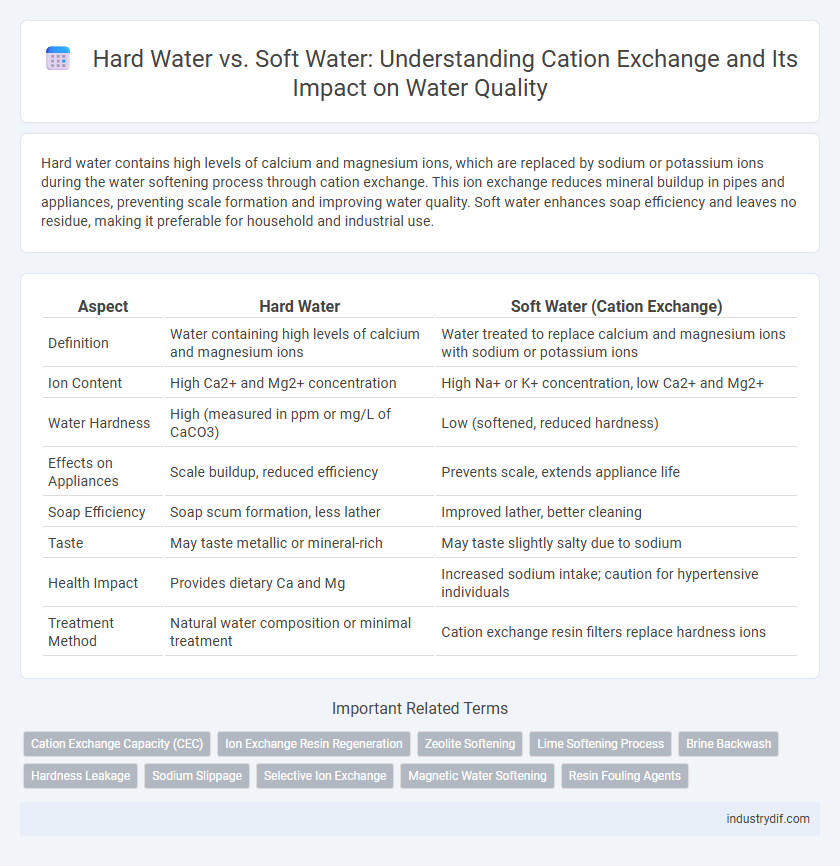
| Aspect | Hard Water | Soft Water (Cation Exchange) |
|---|---|---|
| Definition | Water containing high levels of calcium and magnesium ions | Water treated to replace calcium and magnesium ions with sodium or potassium ions |
| Ion Content | High Ca2+ and Mg2+ concentration | High Na+ or K+ concentration, low Ca2+ and Mg2+ |
| Water Hardness | High (measured in ppm or mg/L of CaCO3) | Low (softened, reduced hardness) |
| Effects on Appliances | Scale buildup, reduced efficiency | Prevents scale, extends appliance life |
| Soap Efficiency | Soap scum formation, less lather | Improved lather, better cleaning |
| Taste | May taste metallic or mineral-rich | May taste slightly salty due to sodium |
| Health Impact | Provides dietary Ca and Mg | Increased sodium intake; caution for hypertensive individuals |
| Treatment Method | Natural water composition or minimal treatment | Cation exchange resin filters replace hardness ions |
Definition of Hard Water and Soft Water
Hard water contains high concentrations of calcium and magnesium ions, resulting from natural mineral deposits in groundwater. Soft water has low levels of these minerals, often due to water softening treatments that replace calcium and magnesium with sodium or potassium ions. The ion exchange process reduces water hardness, improving its effectiveness for cleaning and preventing scale buildup in pipes and appliances.
Causes and Sources of Water Hardness
Water hardness primarily results from high concentrations of calcium and magnesium ions, commonly originating from the dissolution of limestone, chalk, and dolomite in groundwater sources. Hard water forms when these minerals are naturally present as water percolates through sedimentary rock formations, while soft water typically contains fewer dissolved minerals, often sourced from surface water or treated municipal supplies. Ion exchange processes can soften hard water by replacing calcium and magnesium ions with sodium or potassium ions, altering water's mineral composition and reducing scale buildup.
Common Problems Associated with Hard Water
Hard water contains high levels of calcium and magnesium ions, leading to scale buildup in pipes and appliances, reducing their efficiency and lifespan. These minerals interfere with soap's ability to form lather, causing soap scum and skin irritation problems. Water softening through ion exchange replaces calcium and magnesium with sodium or potassium ions, preventing these common hard water issues.
Benefits of Soft Water in Industrial Applications
Soft water enhances industrial equipment longevity by preventing mineral buildup and scale formation that commonly occur with hard water. Its low concentration of calcium and magnesium ions improves the efficiency of boilers, cooling towers, and cleaning processes, reducing maintenance costs and downtime. Industries benefit from soft water's ability to optimize detergent performance and reduce energy consumption during heating operations.
Understanding Cation Exchange Process
Cation exchange in water involves the replacement of hardness-causing calcium (Ca2+) and magnesium (Mg2+) ions with sodium (Na+) or potassium (K+) ions, transforming hard water into soft water. This process occurs in ion exchange resins, which contain negatively charged sites that attract and bind positively charged cations, facilitating the removal of hardness ions. Understanding the cation exchange mechanism is essential for optimizing water softening systems and improving water quality for household and industrial use.
Ion Exchange Resins in Water Softening
Ion exchange resins are crucial in water softening as they replace hardness ions like calcium (Ca2+) and magnesium (Mg2+) with sodium (Na+) or potassium (K+) ions, effectively transforming hard water into soft water. These synthetic polymers contain charged sites that attract and bind hard water ions, preventing scale buildup and improving water quality. Efficient ion exchange resins enhance the longevity of plumbing systems and appliances by reducing lime scale and promoting better soap effectiveness.
Comparing Hard Water vs Soft Water Performance
Hard water contains high concentrations of calcium and magnesium ions, which reduce soap lathering and cause scale buildup in pipes and appliances, impairing their efficiency. Soft water, achieved through ion exchange by replacing hardness ions with sodium or potassium ions, improves soap performance and prevents mineral deposits, enhancing cleaning effectiveness and extending appliance lifespan. The ion exchange process in soft water also reduces corrosion and helps maintain plumbing systems more efficiently compared to untreated hard water.
Maintenance of Cation Exchange Systems
Regular regeneration of cation exchange systems is essential for maintaining effective removal of hardness ions such as calcium and magnesium in hard water treatment. Proper monitoring of resin bed exhaustion and timely application of regenerants like sodium chloride prevent resin fouling and ensure optimal ion exchange capacity. Routine maintenance extends system lifespan, reduces operational costs, and guarantees consistent soft water output for residential or industrial use.
Environmental Impact of Water Softening
Water softening through ion exchange replaces calcium and magnesium ions in hard water with sodium or potassium ions, reducing scale buildup but increasing sodium levels in wastewater. Elevated sodium concentrations from softened water can disrupt soil structure and harm freshwater ecosystems when discharged untreated. Environmentally sustainable alternatives include salt-free water conditioners and improving wastewater treatment to minimize ecological damage.
Selecting the Right Water Treatment Solution
Hard water contains high levels of calcium and magnesium ions, which cause scale buildup and reduce soap effectiveness, whereas soft water is treated through ion exchange to replace these hardness ions with sodium or potassium ions. Selecting the right water treatment solution depends on water hardness levels, household needs, and potential health impacts, with ion exchange water softeners being the most common method for effective hardness removal. Testing water quality and consulting water treatment professionals ensure optimal system design and performance for long-term protection against scale and improved water quality.
Related Important Terms
Cation Exchange Capacity (CEC)
Cation Exchange Capacity (CEC) measures water's ability to hold and exchange positively charged ions, significantly impacting the interaction between hard water, rich in calcium and magnesium ions, and soft water, which contains fewer of these cations. High CEC in water promotes the retention of essential minerals, influencing water hardness and its suitability for agricultural and industrial applications.
Ion Exchange Resin Regeneration
Ion exchange resin regeneration in water softeners involves replacing accumulated calcium (Ca2+) and magnesium (Mg2+) ions on the resin beads with sodium (Na+) or potassium (K+) ions, restoring the resin's ability to soften hard water. Efficient regeneration cycles ensure optimal removal of hardness minerals, maintaining water quality and extending the lifespan of the ion exchange resin.
Zeolite Softening
Zeolite softening uses ion exchange to replace calcium and magnesium ions in hard water with sodium ions, effectively reducing water hardness. This method prolongs appliance lifespan, prevents scale buildup, and maintains water quality for household and industrial use.
Lime Softening Process
Lime softening removes hardness from water by adding calcium hydroxide, which precipitates calcium and magnesium ions responsible for water hardness through a cation exchange reaction. This process reduces scaling and improves water quality by converting hard water into soft water suitable for industrial and domestic use.
Brine Backwash
Hard water contains high levels of calcium and magnesium ions that cause scale buildup, while soft water is produced through ion exchange processes where these hardness ions are replaced with sodium or potassium ions. Brine backwash is a critical step in water softening systems, flushing the ion exchange resin with a concentrated salt solution to regenerate the resin and restore its capacity to remove hardness ions effectively.
Hardness Leakage
Hard water, rich in calcium and magnesium ions, resists cation exchange processes, causing hardness leakage that reduces water softening efficiency. In contrast, soft water, depleted of these hardness ions through effective ion exchange, prevents scale buildup and improves appliance longevity.
Sodium Slippage
Hard water contains high levels of calcium and magnesium ions that are replaced by sodium ions during cation exchange in water softeners, leading to a phenomenon known as sodium slippage. Sodium slippage occurs when excess sodium ions pass through the resin bed without being exchanged, reducing the softening efficiency and potentially increasing sodium concentrations in the treated water.
Selective Ion Exchange
Selective ion exchange in water treatment targets the removal of hardness-causing ions such as calcium (Ca2+) and magnesium (Mg2+) by replacing them with sodium (Na+) or potassium (K+) ions, effectively converting hard water into soft water. This process enhances water quality by preventing scale buildup in plumbing and appliances while maintaining essential minerals through controlled ion selectivity.
Magnetic Water Softening
Magnetic water softening alters the properties of hard water by using magnetic fields to change calcium and magnesium ion behavior, reducing scale buildup without removing these minerals through traditional ion exchange methods. This eco-friendly process enhances water quality by preventing scale deposition while maintaining essential mineral content, unlike conventional softening that replaces hardness ions with sodium.
Resin Fouling Agents
Hard water contains high concentrations of calcium and magnesium ions that cause resin fouling in ion exchange systems, reducing resin efficiency and lifespan. Soft water, treated through ion exchange resins, removes these fouling agents by replacing hardness ions with sodium or potassium ions, ensuring optimal resin performance and water quality.
Hard Water vs Soft Watercation Exchange Infographic
 industrydif.com
industrydif.com