Chemistry studies the properties, composition, and reactions of matter at the atomic and molecular levels, emphasizing covalent and ionic bonds. Supramolecular chemistry focuses on the interactions between molecules, such as hydrogen bonding, van der Waals forces, and host-guest complexes, to form larger, organized structures. This field enables the design of functional assemblies with applications in drug delivery, molecular recognition, and nanotechnology.
Table of Comparison
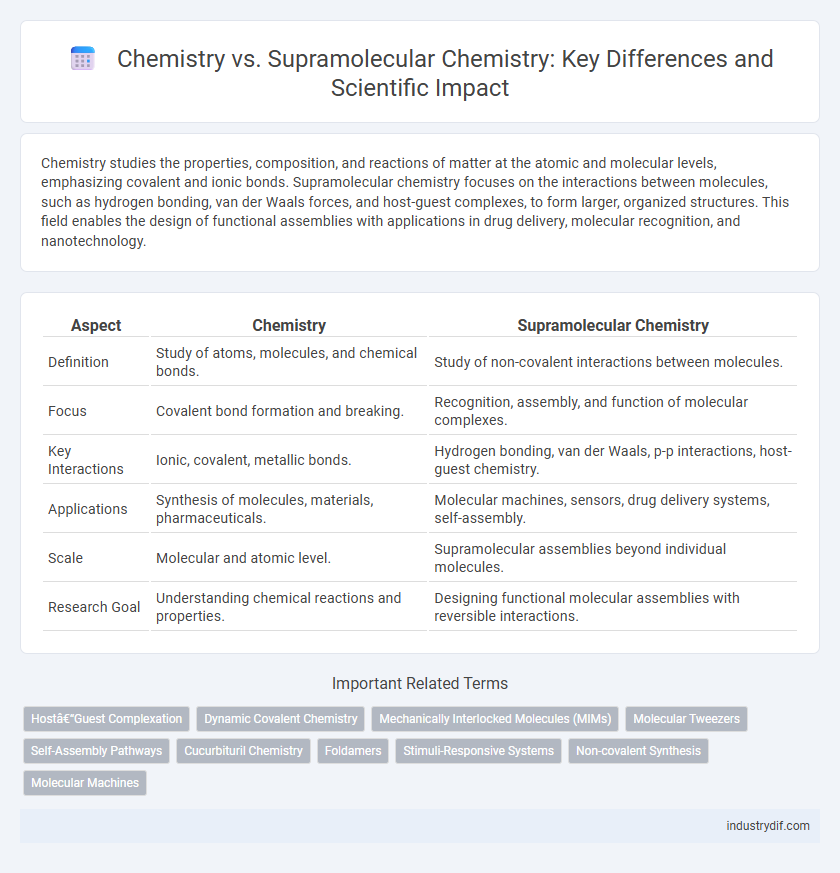
| Aspect | Chemistry | Supramolecular Chemistry |
|---|---|---|
| Definition | Study of atoms, molecules, and chemical bonds. | Study of non-covalent interactions between molecules. |
| Focus | Covalent bond formation and breaking. | Recognition, assembly, and function of molecular complexes. |
| Key Interactions | Ionic, covalent, metallic bonds. | Hydrogen bonding, van der Waals, p-p interactions, host-guest chemistry. |
| Applications | Synthesis of molecules, materials, pharmaceuticals. | Molecular machines, sensors, drug delivery systems, self-assembly. |
| Scale | Molecular and atomic level. | Supramolecular assemblies beyond individual molecules. |
| Research Goal | Understanding chemical reactions and properties. | Designing functional molecular assemblies with reversible interactions. |
Fundamental Principles: Chemistry and Supramolecular Chemistry
Chemistry focuses on the study of atoms, molecules, and their intrinsic chemical bonds, governed by principles such as thermodynamics, kinetics, and molecular structure. Supramolecular chemistry extends these fundamentals by exploring non-covalent interactions like hydrogen bonding, van der Waals forces, and p-p interactions to create complex assemblies and functional systems. Emphasis on molecular recognition and self-assembly processes distinguishes supramolecular chemistry from traditional covalent chemistry, enabling innovations in nanotechnology and materials science.
Molecular Bonds vs. Non-Covalent Interactions
Molecular bonds in traditional chemistry involve covalent or ionic connections where atoms share or transfer electrons, resulting in stable, strong linkages. Supramolecular chemistry focuses on non-covalent interactions such as hydrogen bonding, van der Waals forces, and p-p stacking, which are weaker and reversible, enabling dynamic molecular assemblies. Understanding the distinction between molecular bonds and non-covalent forces is essential for designing functional materials and complex biological systems.
Building Blocks: Atoms, Molecules, and Supramolecular Assemblies
In traditional chemistry, atoms serve as fundamental building blocks that combine to form molecules through covalent bonds, defining molecular structure and reactivity. Supramolecular chemistry transcends this by focusing on non-covalent interactions such as hydrogen bonding, van der Waals forces, and p-p stacking to assemble discrete molecules into functional supramolecular architectures. These supramolecular assemblies exhibit emergent properties distinct from individual molecules, enabling complex functionalities in catalysis, molecular recognition, and materials science.
Structural Hierarchies in Chemistry
Structural hierarchies in traditional chemistry primarily involve atomic and molecular levels, focusing on covalent bonding and discrete molecular entities. Supramolecular chemistry extends beyond these levels by emphasizing non-covalent interactions such as hydrogen bonding, van der Waals forces, and p-p stacking to assemble complex architectures. This hierarchical organization allows for dynamic and reversible assemblies, enabling functional systems with emergent properties unattainable by conventional chemical synthesis.
Host–Guest Chemistry: Supramolecular Recognition
Host-guest chemistry, a key aspect of supramolecular chemistry, focuses on the non-covalent interactions between a host molecule and a guest molecule, enabling selective molecular recognition. Unlike traditional chemistry that primarily deals with covalent bonds, supramolecular chemistry exploits hydrogen bonding, van der Waals forces, and p-p interactions to form reversible and dynamic complexes. This field drives advancements in molecular sensors, drug delivery systems, and nanotechnology by mimicking biological recognition processes.
Analytical Methods in Chemistry and Supramolecular Chemistry
Analytical methods in traditional chemistry primarily focus on identifying and quantifying molecular species using techniques such as chromatography, mass spectrometry, and NMR spectroscopy. In supramolecular chemistry, analytical approaches emphasize characterizing non-covalent interactions and host-guest complexes through methods like isothermal titration calorimetry, fluorescence spectroscopy, and atomic force microscopy. Advanced spectroscopy and microscopy techniques enable detailed insights into the dynamic behavior and structural organization of supramolecular assemblies, which are essential for understanding their functional properties.
Material Applications: From Molecules to Supramolecular Systems
Chemistry traditionally focuses on the synthesis and properties of individual molecules, while supramolecular chemistry explores the non-covalent interactions that organize molecules into larger, functional assemblies. Material applications span from designing molecular catalysts and sensors to creating supramolecular polymers and nanostructures with enhanced mechanical, optical, and electronic properties. The shift from molecules to supramolecular systems enables the development of adaptive materials with dynamic, self-healing, and stimuli-responsive capabilities for advanced technological uses.
Functionalities: Reactivity versus Organization
Chemistry primarily emphasizes reactivity, focusing on the making and breaking of covalent bonds to form new molecular entities with specific functions. Supramolecular chemistry explores the organization of molecules through non-covalent interactions like hydrogen bonding, p-p stacking, and van der Waals forces, enabling the formation of complex architectures with dynamic and reversible functionalities. This shift from reactivity to organization facilitates the development of responsive materials, molecular recognition systems, and self-assembled nanostructures with programmable behaviors.
Chemical Synthesis vs. Supramolecular Self-Assembly
Chemical synthesis involves the construction of molecules through covalent bond formation, allowing precise control over molecular structure and function. Supramolecular self-assembly relies on non-covalent interactions such as hydrogen bonding, metal coordination, and van der Waals forces to form organized architectures spontaneously. This approach enables dynamic and reversible formation of complex systems, distinguishing it from traditional synthetic methods focused on permanent molecular bonds.
Future Trends in Chemistry and Supramolecular Chemistry
Future trends in chemistry emphasize sustainable synthesis, advanced catalysis, and smart materials, while supramolecular chemistry drives innovation through programmable self-assembly, molecular recognition, and dynamic systems. Integration of AI and machine learning accelerates discovery processes by predicting molecular interactions and optimizing complex structures. The convergence of these fields fosters development in targeted drug delivery, environmentally friendly catalysts, and responsive nanomaterials for diverse technological applications.
Related Important Terms
Host–Guest Complexation
Chemistry traditionally studies molecular interactions and reactions at the atomic level, while supramolecular chemistry emphasizes non-covalent interactions crucial for host-guest complexation, enabling selective molecular recognition and reversible binding. Host-guest complexation involves tailored host molecules forming stable yet dynamic assemblies with guest species through hydrogen bonding, van der Waals forces, and electrostatic interactions, advancing applications in molecular sensing, drug delivery, and catalysis.
Dynamic Covalent Chemistry
Dynamic covalent chemistry bridges traditional chemistry and supramolecular chemistry by enabling reversible covalent bond formation under thermodynamic control, fostering error correction and self-assembly of complex molecular architectures. Unlike static covalent bonds in classical chemistry, dynamic covalent bonds allow adaptive and stimuli-responsive molecular systems essential for developing smart materials and molecular machines.
Mechanically Interlocked Molecules (MIMs)
Mechanically Interlocked Molecules (MIMs) represent a distinct class of compounds within supramolecular chemistry characterized by their topological bonds, such as catenanes and rotaxanes, which differ fundamentally from traditional covalent bonding found in classical chemistry. These MIMs enable the study of molecular machines and dynamic functions, leveraging non-covalent interactions to create mechanically linked structures with potential applications in nanotechnology and molecular electronics.
Molecular Tweezers
Molecular tweezers in supramolecular chemistry function as non-covalent host molecules that selectively bind guest species through hydrogen bonding, p-p interactions, and van der Waals forces, contrasting traditional covalent bond formation in classical chemistry. These synthetic receptors enable precise molecular recognition and reversible complexation, facilitating advancements in drug delivery, molecular sensing, and nanotechnology.
Self-Assembly Pathways
Supramolecular chemistry explores self-assembly pathways driven by non-covalent interactions such as hydrogen bonding, p-p stacking, and metal coordination, enabling dynamic and reversible formation of complex structures. In contrast, traditional chemistry primarily relies on covalent bond formation, which leads to more rigid and static assemblies with limited adaptability in molecular organization.
Cucurbituril Chemistry
Cucurbituril chemistry, a key area within supramolecular chemistry, involves the study of macrocyclic molecules that form highly selective host-guest complexes through non-covalent interactions, differing fundamentally from traditional chemistry's focus on covalent bonding and molecular synthesis. The unique ability of cucurbiturils to encapsulate ions and neutral molecules with exceptional binding affinity has catalyzed advances in drug delivery, molecular recognition, and catalysis, distinguishing supramolecular approaches from conventional chemical methodologies.
Foldamers
Foldamers are synthetic oligomers that mimic the folding patterns of natural biomolecules, bridging traditional chemistry and supramolecular chemistry by emphasizing non-covalent interactions for structural control. Supramolecular chemistry exploits hydrogen bonding, p-p stacking, and metal coordination to design foldamers with precise conformations, enhancing their potential in molecular recognition, catalysis, and materials science.
Stimuli-Responsive Systems
Chemistry traditionally studies molecular interactions and transformations, whereas supramolecular chemistry emphasizes non-covalent interactions to create stimuli-responsive systems that undergo reversible structural changes upon exposure to light, temperature, pH, or chemical agents. These dynamic assemblies enable precise control over material properties and functions, critical for applications in drug delivery, sensing, and smart materials.
Non-covalent Synthesis
Non-covalent synthesis in supramolecular chemistry exploits intermolecular forces such as hydrogen bonding, van der Waals interactions, and electrostatics to create complex assemblies, distinguishing it from traditional covalent bond formation in classical chemistry. This approach enables reversible, self-assembling systems with dynamic properties critical for molecular recognition, catalysis, and nanotechnology applications.
Molecular Machines
Chemistry traditionally investigates the properties, structures, and reactions of molecules, while supramolecular chemistry explores the non-covalent interactions and self-assembly processes that enable the construction of complex molecular machines. These molecular machines operate through precise coordination of components at the nanoscale, harnessing dynamic supramolecular interactions to perform specific mechanical tasks in response to external stimuli.
Chemistry vs Supramolecular Chemistry Infographic
 industrydif.com
industrydif.com