Classical thermodynamics analyzes systems in equilibrium, focusing on macroscopic properties such as temperature, pressure, and entropy to describe energy transformations. Non-equilibrium thermodynamics studies systems away from equilibrium, addressing irreversible processes and fluxes that drive changes in time and space. Understanding the distinctions between these frameworks enhances insight into complex physical and biological phenomena beyond static states.
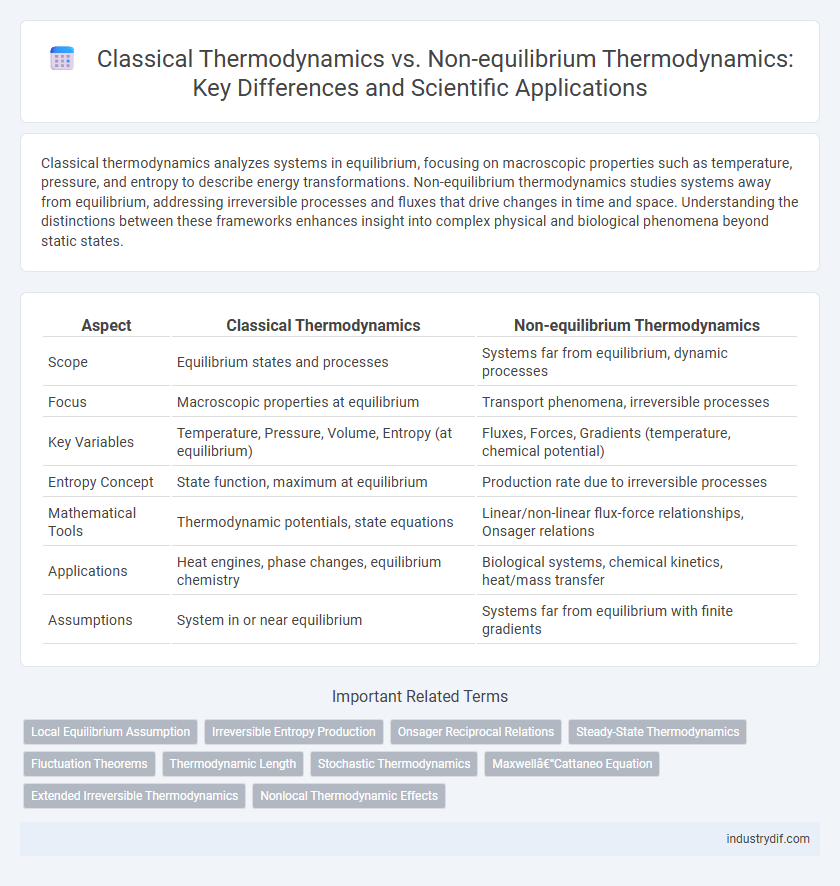
Table of Comparison
| Aspect | Classical Thermodynamics | Non-equilibrium Thermodynamics |
|---|---|---|
| Scope | Equilibrium states and processes | Systems far from equilibrium, dynamic processes |
| Focus | Macroscopic properties at equilibrium | Transport phenomena, irreversible processes |
| Key Variables | Temperature, Pressure, Volume, Entropy (at equilibrium) | Fluxes, Forces, Gradients (temperature, chemical potential) |
| Entropy Concept | State function, maximum at equilibrium | Production rate due to irreversible processes |
| Mathematical Tools | Thermodynamic potentials, state equations | Linear/non-linear flux-force relationships, Onsager relations |
| Applications | Heat engines, phase changes, equilibrium chemistry | Biological systems, chemical kinetics, heat/mass transfer |
| Assumptions | System in or near equilibrium | Systems far from equilibrium with finite gradients |
Introduction to Thermodynamic Frameworks
Classical thermodynamics primarily addresses systems at equilibrium, utilizing state functions such as entropy, enthalpy, and free energy to describe macroscopic properties and predict spontaneous processes. Non-equilibrium thermodynamics extends this framework to systems away from equilibrium by incorporating fluxes, forces, and time-dependent variables, enabling the analysis of irreversible processes and transport phenomena. Both frameworks are essential for understanding energy transformations, with classical thermodynamics providing foundational principles and non-equilibrium thermodynamics offering detailed insights into dynamic behaviors in diverse scientific and engineering applications.
Defining Classical Thermodynamics
Classical thermodynamics is the study of macroscopic systems in thermodynamic equilibrium, focusing on state variables such as temperature, pressure, and volume, and their interrelations through laws like the first and second law of thermodynamics. It assumes that the system is homogeneous and time-independent, allowing the use of equilibrium state functions to describe energy transformations and entropy changes. This framework does not account for irreversible processes or spatial and temporal gradients, which are central to non-equilibrium thermodynamics.
Fundamentals of Non-equilibrium Thermodynamics
Non-equilibrium thermodynamics extends classical thermodynamics by addressing systems far from equilibrium, where fluxes and forces drive irreversible processes. It incorporates concepts such as entropy production, linear phenomenological laws, and Onsager reciprocal relations to describe transport phenomena and relaxation towards equilibrium. This framework enables the quantitative analysis of energy dissipation, chemical reactions, and heat and mass transfer in complex, time-dependent systems beyond the scope of classical equilibrium assumptions.
Key Laws and Principles: Comparison
Classical Thermodynamics primarily relies on the four fundamental laws--including the Zeroth, First, Second, and Third Laws--that govern systems at equilibrium, emphasizing state functions and reversible processes. Non-equilibrium Thermodynamics extends these principles to systems away from equilibrium, incorporating concepts like entropy production, fluxes, and forces to describe irreversible processes. The key distinction lies in how entropy behavior is treated: classical thermodynamics assumes constant entropy in reversible changes, whereas non-equilibrium thermodynamics quantifies entropy generation during spontaneous, irreversible transformations.
State Variables and Thermodynamic Potentials
Classical thermodynamics primarily deals with equilibrium states, utilizing state variables such as temperature, pressure, and volume to define thermodynamic potentials like internal energy, enthalpy, Helmholtz free energy, and Gibbs free energy. Non-equilibrium thermodynamics extends these concepts by incorporating fluxes and gradients of state variables to describe systems away from equilibrium, often requiring generalized potentials and additional state variables that account for irreversible processes. The distinction lies in classical thermodynamics relying on well-defined state functions for closed systems at equilibrium, while non-equilibrium thermodynamics addresses dynamic changes through extended state spaces and time-dependent potentials.
Irreversibility and Entropy Production
Classical thermodynamics primarily addresses systems at or near equilibrium, emphasizing reversible processes where entropy remains constant. Non-equilibrium thermodynamics extends this framework to analyze irreversible processes, highlighting the continuous production of entropy as systems evolve towards equilibrium. Entropy production serves as a key measure of irreversibility, quantifying energy dissipation and the unidirectional flow of natural processes in thermodynamic systems.
Transport Processes: Diffusion, Heat, Mass
Classical thermodynamics primarily addresses systems at equilibrium, where transport processes such as diffusion, heat, and mass transfer occur without gradients, limiting its applicability to steady-state conditions. Non-equilibrium thermodynamics extends this framework by incorporating fluxes driven by gradients in temperature, concentration, and chemical potential, thus providing a quantitative description of irreversible processes in open systems. Transport coefficients like thermal conductivity, diffusivity, and mass transfer coefficients are key parameters that link thermodynamic forces to fluxes, enabling precise modeling of real-world phenomena such as heat conduction, molecular diffusion, and convective mass transfer.
Applications in Modern Science and Engineering
Classical thermodynamics provides foundational principles for analyzing energy conversion and equilibrium states in engineering systems such as heat engines and refrigeration cycles. Non-equilibrium thermodynamics extends these principles to quantify irreversible processes, enabling advanced modeling of transport phenomena, chemical reactions, and biological systems under dynamic conditions. Applications in modern science include optimizing energy efficiency in renewable technologies and understanding complex materials behavior far from equilibrium.
Limitations and Challenges of Each Approach
Classical thermodynamics primarily addresses systems at or near equilibrium, limiting its applicability to dynamic processes where gradients and fluxes dominate behavior. Non-equilibrium thermodynamics extends analysis to irreversible processes and spatial-temporal variations but faces challenges in formulating universal governing equations and handling complex, nonlinear systems. Both approaches struggle with multiscale interactions, requiring advanced models to accurately predict real-world system behavior beyond idealized conditions.
Future Perspectives in Thermodynamic Analysis
Future perspectives in thermodynamic analysis emphasize integrating non-equilibrium thermodynamics with classical principles to enhance the understanding of complex, real-world systems far from equilibrium. Advanced computational models and experimental techniques aim to quantify entropy production and energy dissipation at micro and nanoscale levels, enabling the design of more efficient energy conversion processes. Emerging frameworks focus on bridging scales from molecular dynamics to continuum descriptions, promoting innovations in sustainable energy, materials science, and biological systems analysis.
Related Important Terms
Local Equilibrium Assumption
Classical thermodynamics relies on the local equilibrium assumption, presuming that thermodynamic variables are well-defined and uniform within infinitesimal regions despite the presence of gradients, enabling the use of equilibrium state functions. Non-equilibrium thermodynamics extends beyond this assumption by addressing systems with significant spatial and temporal gradients where local equilibrium may not hold, incorporating fluxes and irreversible processes into its formulations.
Irreversible Entropy Production
Classical thermodynamics primarily addresses systems at or near equilibrium, where entropy production is negligible or zero, while non-equilibrium thermodynamics focuses on irreversible processes characterized by positive entropy production due to gradients in temperature, chemical potential, or other thermodynamic forces. Irreversible entropy production quantifies the dissipation of energy and the deviation from equilibrium, serving as a fundamental parameter in describing the direction and efficiency of real-world thermodynamic processes.
Onsager Reciprocal Relations
Onsager Reciprocal Relations establish a symmetrical connection between fluxes and forces in near-equilibrium systems, providing foundational equations that extend classical thermodynamics into non-equilibrium thermodynamics. These relations quantify linear phenomenological coefficients, enabling precise prediction of irreversible processes and energy dissipation beyond equilibrium constraints.
Steady-State Thermodynamics
Classical Thermodynamics primarily addresses systems in equilibrium, characterizing state variables without considering fluxes or temporal changes, whereas Non-equilibrium Thermodynamics extends these principles to analyze energy and matter exchanges in systems far from equilibrium. Steady-State Thermodynamics specifically focuses on systems maintaining constant macroscopic properties over time despite continuous fluxes, enabling the study of irreversible processes under sustained non-equilibrium conditions.
Fluctuation Theorems
Classical thermodynamics describes macroscopic systems at equilibrium, relying on laws such as the second law to predict irreversible processes, whereas non-equilibrium thermodynamics extends these principles to systems far from equilibrium, incorporating time-dependent phenomena and fluxes. Fluctuation theorems quantitatively characterize the probability distributions of entropy production in non-equilibrium systems, providing a fundamental link between microscopic reversibility and macroscopic irreversibility beyond the traditional thermodynamic framework.
Thermodynamic Length
Thermodynamic length quantifies the efficiency of state transformations by measuring the distance in parameter space, with classical thermodynamics typically addressing equilibrium states and reversible processes, while non-equilibrium thermodynamics extends this concept to irreversible processes and finite-time transformations. Understanding thermodynamic length in non-equilibrium contexts enables optimization of energy dissipation and better control of real-world thermodynamic systems away from equilibrium.
Stochastic Thermodynamics
Classical thermodynamics primarily describes macroscopic systems at or near equilibrium using deterministic state variables, whereas non-equilibrium thermodynamics extends these principles to systems driven far from equilibrium, often incorporating fluxes and forces. Stochastic thermodynamics provides a probabilistic framework to analyze fluctuations and energy exchanges at microscopic scales, enabling quantitative insights into molecular machines and nanoscale processes beyond traditional thermodynamic limits.
Maxwell–Cattaneo Equation
The Maxwell-Cattaneo equation addresses the limitations of Fourier's law in classical thermodynamics by incorporating finite-speed heat propagation, thus resolving the paradox of infinite thermal propagation speed. This equation plays a crucial role in non-equilibrium thermodynamics by modeling heat flux relaxation and transient heat conduction phenomena.
Extended Irreversible Thermodynamics
Classical Thermodynamics primarily addresses systems at equilibrium, focusing on macroscopic properties and state functions, whereas Non-equilibrium Thermodynamics, particularly Extended Irreversible Thermodynamics (EIT), extends the framework to describe irreversible processes using additional fluxes and internal variables beyond local equilibrium assumptions. EIT incorporates nonlinear transport equations and memory effects, enabling accurate modeling of transient phenomena and complex irreversible behaviors in systems far from equilibrium.
Nonlocal Thermodynamic Effects
Non-equilibrium thermodynamics extends classical thermodynamics by incorporating spatial and temporal nonlocalities, allowing accurate modeling of systems with gradients and fluxes far from equilibrium states. Nonlocal thermodynamic effects capture interactions beyond immediate neighbors, enabling analysis of complex phenomena such as heat conduction with memory and spatial correlations in heterogeneous materials.
Classical Thermodynamics vs Non-equilibrium Thermodynamics Infographic
 industrydif.com
industrydif.com