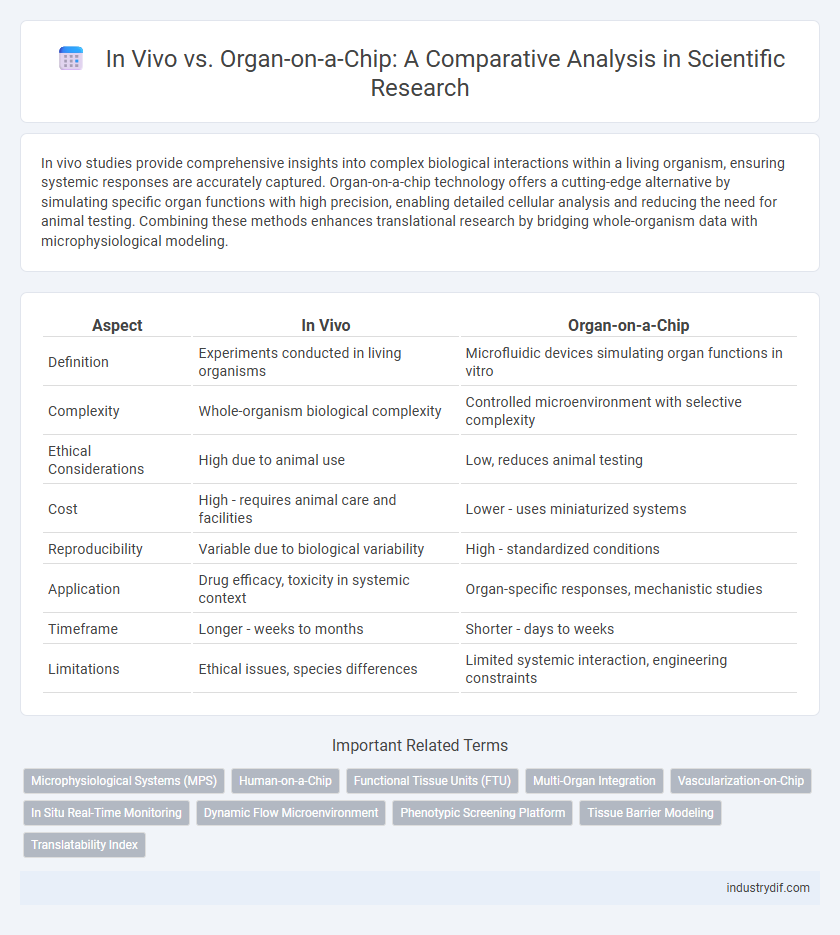
In vivo studies provide comprehensive insights into complex biological interactions within a living organism, ensuring systemic responses are accurately captured. Organ-on-a-chip technology offers a cutting-edge alternative by simulating specific organ functions with high precision, enabling detailed cellular analysis and reducing the need for animal testing. Combining these methods enhances translational research by bridging whole-organism data with microphysiological modeling.
Table of Comparison
| Aspect | In Vivo | Organ-on-a-Chip |
|---|---|---|
| Definition | Experiments conducted in living organisms | Microfluidic devices simulating organ functions in vitro |
| Complexity | Whole-organism biological complexity | Controlled microenvironment with selective complexity |
| Ethical Considerations | High due to animal use | Low, reduces animal testing |
| Cost | High - requires animal care and facilities | Lower - uses miniaturized systems |
| Reproducibility | Variable due to biological variability | High - standardized conditions |
| Application | Drug efficacy, toxicity in systemic context | Organ-specific responses, mechanistic studies |
| Timeframe | Longer - weeks to months | Shorter - days to weeks |
| Limitations | Ethical issues, species differences | Limited systemic interaction, engineering constraints |
Introduction to In Vivo and Organ-on-a-Chip Technologies
In vivo studies involve testing within a living organism, providing comprehensive biological context but often facing ethical and logistical challenges. Organ-on-a-chip technology mimics organ-level functions on microfluidic devices, enabling precise control of cellular environments and real-time monitoring. These platforms offer a promising alternative to traditional animal models by improving physiological relevance and reducing experimental variability.
Historical Evolution of Biological Testing Methods
The historical evolution of biological testing methods transitioned from traditional in vivo experiments, relying on whole-animal models to study physiological responses, to the development of organ-on-a-chip technology, which mimics human organ functions using microfluidic systems. Organ-on-a-chip devices emerged as a breakthrough in the early 21st century, offering enhanced precision and reduced ethical concerns compared to animal testing. Advances in microengineering and cell biology have driven this shift, enabling more accurate disease modeling and drug testing with improved relevance to human physiology.
Defining In Vivo Studies: Principles and Applications
In vivo studies involve conducting experiments within living organisms to observe biological processes in their natural environment, providing comprehensive insights into complex physiological interactions. These studies are fundamental in drug development, toxicology, and disease modeling due to their ability to replicate systemic responses and metabolic pathways accurately. Key applications include evaluating pharmacokinetics, assessing therapeutic efficacy, and understanding pathophysiological mechanisms in whole-animal models.
Organ-on-a-Chip: Technology Overview and Key Components
Organ-on-a-chip technology mimics human physiological functions using microfluidic devices that integrate living cells within a controlled environment. Key components include microchannels for fluid flow, sensors for monitoring biochemical and mechanical cues, and scaffolds that replicate tissue architecture. This platform enables precise modeling of organ-level responses, improving drug testing and disease research accuracy compared to traditional in vivo models.
Comparative Analysis: In Vivo Versus Organ-on-a-Chip Models
In vivo models provide comprehensive physiological context by preserving systemic interactions within a living organism, critical for studying complex disease mechanisms and drug metabolism. Organ-on-a-chip platforms replicate specific organ microenvironments with precise control over cellular architecture and mechanical forces, enhancing the modeling of organ-specific responses and reducing variability. Comparative analyses reveal that organ-on-a-chip systems offer higher throughput and ethical advantages while in vivo models remain essential for evaluating whole-body pharmacokinetics and immune system dynamics.
Advantages and Limitations of In Vivo Approaches
In vivo approaches provide comprehensive biological context by studying complex interactions within a whole organism, which allows for accurate assessment of systemic effects and long-term outcomes. These methods benefit from well-established protocols and regulatory acceptance, making them critical for drug development and toxicity testing. However, limitations include high costs, ethical concerns, species differences that may compromise human relevance, and limited ability to isolate specific cellular mechanisms compared to organ-on-a-chip models.
Benefits and Challenges of Organ-on-a-Chip Systems
Organ-on-a-chip systems offer precise control over microenvironments, enabling more accurate simulation of human organ functions compared to traditional in vivo models. These platforms reduce reliance on animal testing and allow real-time monitoring of cellular responses, enhancing drug discovery and toxicity testing. Challenges include replicating complex systemic interactions and ensuring scalability for widespread pharmaceutical application.
Regulatory Perspectives and Validation Standards
Regulatory agencies increasingly emphasize the validation of organ-on-a-chip models to ensure predictive accuracy and reproducibility compared to traditional in vivo studies, enhancing their acceptance for drug development and toxicity testing. Validation standards, such as those outlined by the FDA and EMA, focus on the biological relevance, mechanistic modeling, and data robustness of these microphysiological systems. Bridging these regulatory frameworks with in vivo data supports accelerated adoption of organ-on-a-chip technologies while meeting stringent safety and efficacy requirements.
Recent Advances and Breakthroughs in In Vivo and Organ-on-a-Chip Research
Recent advances in in vivo research have enhanced precision in disease modeling through CRISPR gene-editing and high-resolution imaging techniques, enabling real-time monitoring of cellular processes in living organisms. Organ-on-a-chip technology has achieved breakthroughs in replicating complex human organ microenvironments using microfluidic systems, integrating sensors for dynamic biochemical analysis and improved drug response prediction. Combining these innovations accelerates translational research by bridging physiological relevance with controlled experimental conditions, offering superior insights into pathophysiology and therapeutic development.
Future Trends and Industry Impact of Preclinical Testing Models
Organ-on-a-chip technology is rapidly advancing, offering more precise simulation of human physiology compared to traditional in vivo models, thereby reducing reliance on animal testing and improving predictive accuracy for drug efficacy and toxicity. Integration of microfluidics, 3D cell cultures, and real-time biosensing is driving enhanced data fidelity and scalability in preclinical testing. Future trends indicate widespread adoption of organ-on-a-chip platforms in pharmaceutical development, accelerating personalized medicine and regulatory acceptance while lowering overall costs and ethical concerns associated with in vivo experiments.
Related Important Terms
Microphysiological Systems (MPS)
Microphysiological Systems (MPS) offer a cutting-edge alternative to traditional in vivo models by replicating human tissue architecture and function on microfluidic chips, enhancing predictive accuracy in drug development. These organ-on-a-chip platforms integrate multiple cell types and dynamic microenvironments to simulate organ-level physiology, reducing reliance on animal testing and improving translational relevance for human health outcomes.
Human-on-a-Chip
Human-on-a-chip technology integrates multiple organ-on-a-chip systems to replicate complex human physiological responses more accurately than traditional in vivo models, enhancing drug development and toxicity testing precision. This approach utilizes microfluidic platforms to simulate systemic interactions, offering scalable and ethical alternatives for personalized medicine research.
Functional Tissue Units (FTU)
Functional Tissue Units (FTUs) in In Vivo models represent complex, multicellular structures with dynamic physiological interactions that closely mimic natural tissue environments, whereas Organ-on-a-chip systems engineer microfluidic platforms integrating FTUs to recreate precise tissue functions and mechanical stimuli in vitro. These chip-based FTUs enable controlled experimental manipulation and high-throughput analysis, advancing the understanding of tissue-specific responses and drug efficacy beyond traditional animal models.
Multi-Organ Integration
Multi-organ integration in organ-on-a-chip systems enables precise simulation of inter-organ interactions and systemic physiological responses that are challenging to replicate in traditional in vivo models. These microfluidic platforms provide controlled environments facilitating dynamic biochemical communication between tissue constructs, enhancing predictive accuracy for drug metabolism and toxicity studies compared to whole-animal experiments.
Vascularization-on-Chip
Vascularization-on-chip models enhance the physiological relevance of organ-on-a-chip systems by replicating microvascular networks that support nutrient and oxygen transport, closely mimicking in vivo blood vessel dynamics. These platforms enable precise control over endothelial cell behavior, shear stress, and biochemical gradients, facilitating advanced studies in angiogenesis, vascular permeability, and drug delivery mechanisms compared to traditional in vivo models.
In Situ Real-Time Monitoring
In vivo models provide comprehensive physiological relevance for studying biological processes within living organisms, yet organ-on-a-chip technologies offer advanced in situ real-time monitoring capabilities through integrated sensors and microfluidic control, enabling precise manipulation and analysis at the cellular level. These microengineered platforms facilitate continuous data acquisition on cellular responses, biochemical changes, and tissue dynamics, surpassing traditional in vivo limitations in temporal and spatial resolution.
Dynamic Flow Microenvironment
Dynamic flow microenvironments in organ-on-a-chip systems replicate physiological shear stress and nutrient gradients more accurately than traditional in vivo models, enabling precise control over cellular microenvironments and real-time monitoring of tissue responses. This microfluidic technology enhances the fidelity of experimental results by mimicking vascular flow dynamics, improving the relevance of preclinical studies in drug development and disease modeling.
Phenotypic Screening Platform
In vivo phenotypic screening platforms provide comprehensive biological context by studying living organisms, capturing systemic interactions essential for accurate drug response evaluation. Organ-on-a-chip technology offers a high-throughput alternative that replicates organ-level microenvironments with precise control over variables, enhancing the predictive power of phenotypic assays while reducing ethical concerns associated with animal testing.
Tissue Barrier Modeling
Tissue barrier modeling in vivo provides a complex, physiologically relevant environment that captures systemic interactions but often lacks precise control over experimental variables. Organ-on-a-chip platforms offer microscale tissue barrier models with tunable parameters, enabling real-time monitoring of cellular responses and more reproducible studies of barrier integrity under controlled mechanical and chemical stimuli.
Translatability Index
Organ-on-a-chip platforms demonstrate a higher Translatability Index compared to traditional in vivo models by closely mimicking human physiological and pathological conditions at the microscale, leading to improved predictive accuracy in drug efficacy and toxicity assessments. Quantitative analyses reveal organ-on-a-chip systems reduce species-specific variability inherent in in vivo studies, enhancing clinical relevance and accelerating translational research outcomes.
In Vivo vs Organ-on-a-chip Infographic
 industrydif.com
industrydif.com