Microscopy allows scientists to observe specimens at the cellular level with standard light resolution limits, typically around 200 nanometers. Super-resolution microscopy surpasses these constraints, providing detailed imaging at the nanometer scale and revealing structures within cells that are otherwise invisible. This advanced technique enhances the understanding of complex biological processes by enabling visualization beyond the diffraction limit of traditional microscopy.
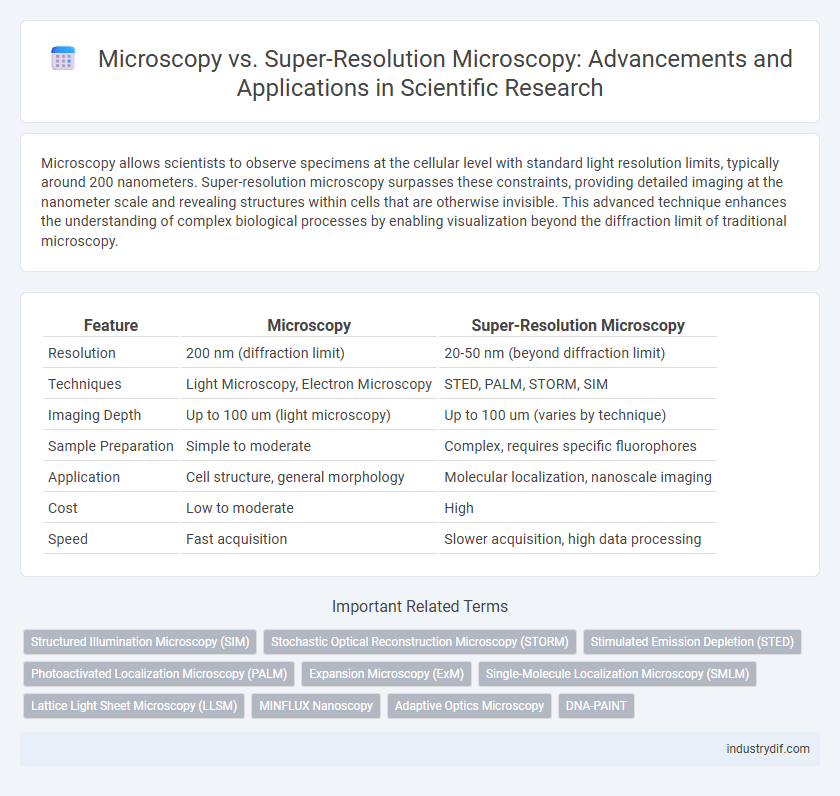
Table of Comparison
| Feature | Microscopy | Super-Resolution Microscopy |
|---|---|---|
| Resolution | 200 nm (diffraction limit) | 20-50 nm (beyond diffraction limit) |
| Techniques | Light Microscopy, Electron Microscopy | STED, PALM, STORM, SIM |
| Imaging Depth | Up to 100 um (light microscopy) | Up to 100 um (varies by technique) |
| Sample Preparation | Simple to moderate | Complex, requires specific fluorophores |
| Application | Cell structure, general morphology | Molecular localization, nanoscale imaging |
| Cost | Low to moderate | High |
| Speed | Fast acquisition | Slower acquisition, high data processing |
Defining Microscopy: Fundamental Concepts
Microscopy involves the use of optical instruments to magnify and resolve images of small objects beyond the capabilities of the naked eye, typically limited by the diffraction limit of light (~200 nm). Super-resolution microscopy techniques, such as STED, PALM, and STORM, overcome this diffraction barrier by employing advanced illumination and image reconstruction methods, achieving resolutions down to 20 nm or less. Fundamental concepts of microscopy include light-matter interaction, optical resolution, and contrast mechanisms, which are critical for interpreting biological and material structures at the nanoscale.
Introduction to Super-Resolution Microscopy
Super-resolution microscopy surpasses the diffraction limit of conventional light microscopy, enabling visualization of cellular structures at nanometer resolution. Techniques such as STED, PALM, and STORM exploit fluorescent molecules and advanced optics to achieve detailed imaging beyond the 200 nm barrier. This breakthrough facilitates unprecedented insights into molecular interactions and dynamic processes within living cells.
Resolution Limits in Conventional Microscopy
Conventional microscopy is limited by the diffraction of light, which restricts resolution to approximately 200 nanometers laterally and 500 nanometers axially, preventing the visualization of structures smaller than these thresholds. This diffraction limit, defined by Ernst Abbe's equation, constrains the ability to observe nanoscale biological components and molecular processes in detail. Super-resolution microscopy techniques, such as STED, PALM, and STORM, overcome these limits by employing fluorescence manipulation and computational reconstruction to achieve resolutions beyond the diffraction barrier, enabling visualization at the tens of nanometers scale.
Breaking the Diffraction Barrier: Super-Resolution Techniques
Super-resolution microscopy surpasses the diffraction limit of conventional optical microscopy, achieving resolutions below 200 nanometers by utilizing techniques such as STED, PALM, and STORM. These methods exploit fluorescence photoactivation, depletion, and localization to image cellular structures at the nanoscale with unprecedented clarity. Breaking the diffraction barrier enables detailed visualization of molecular processes previously inaccessible to standard microscopy, revolutionizing cell biology and nanotechnology research.
Key Technologies in Super-Resolution Microscopy
Super-resolution microscopy leverages advanced technologies such as stimulated emission depletion (STED), structured illumination microscopy (SIM), and single-molecule localization microscopy (SMLM) techniques like PALM and STORM to surpass the diffraction limit of conventional microscopy. These methods rely on precise control of fluorescence emission and localization at the nanoscale, enabling visualization of subcellular structures with resolution down to 10-20 nanometers. Integration of adaptive optics and advanced computational algorithms further enhances image clarity and resolution in super-resolution microscopy.
Applications: Conventional vs. Super-Resolution Microscopy
Conventional microscopy is widely used in biological research for observing cell morphology and tissue architecture with resolution limited by the diffraction of light, typically around 200 nanometers. Super-resolution microscopy techniques such as STED, PALM, and STORM overcome this diffraction limit, enabling visualization of molecular interactions and subcellular structures at nanometer-scale resolution. Applications of super-resolution microscopy include detailed imaging of protein localization, intracellular dynamics, and nanoscale cellular components, which are critical for advancing understanding in cell biology and molecular medicine.
Sample Preparation and Imaging Protocols
Sample preparation for conventional microscopy typically involves standard fixation and staining procedures compatible with widefield or confocal imaging, while super-resolution microscopy requires specialized protocols such as fluorophore selection and optimization to enhance resolution beyond the diffraction limit. Imaging protocols in super-resolution techniques like STED, PALM, or STORM demand precise control of illumination patterns, photoactivation, and data acquisition timing to reconstruct high-resolution images, in contrast to the relatively straightforward acquisition settings of traditional microscopy. The increased complexity in sample preparation and imaging protocols for super-resolution microscopy is critical for achieving nanometer-scale spatial resolution and accurate molecular localization.
Data Analysis and Imaging Artifacts
Microscopy data analysis involves resolving spatial details limited by the diffraction barrier, where conventional techniques often struggle to distinguish closely spaced structures. Super-resolution microscopy employs advanced algorithms and point-spread function modeling to enhance image resolution beyond the diffraction limit, enabling precise quantification of molecular distributions. Imaging artifacts such as photobleaching, background noise, and reconstruction errors are mitigated through optimized data processing pipelines and calibration standards unique to super-resolution methods.
Challenges and Limitations in Microscopy Methods
Conventional microscopy faces resolution limits due to the diffraction barrier, restricting detailed visualization of subcellular structures below approximately 200 nanometers. Photobleaching and limited contrast pose significant challenges in fluorescence microscopy, hindering long-term imaging and accurate quantification. Super-resolution microscopy techniques overcome resolution constraints but are limited by complex sample preparation, high computational demands, and slower imaging speeds, impacting real-time biological studies.
Future Trends in Microscopy Technology
Advancements in microscopy technology are rapidly shifting toward super-resolution techniques that overcome the diffraction limit of traditional light microscopy, enabling visualization of cellular structures at the nanoscale. Emerging trends include integrating artificial intelligence for enhanced image processing and real-time data analysis, along with the development of hybrid systems combining super-resolution with cryo-electron microscopy to achieve unprecedented spatial resolution. Future microscopy platforms are expected to provide deeper insights into dynamic biological processes, facilitating breakthroughs in molecular biology, neurobiology, and clinical diagnostics.
Related Important Terms
Structured Illumination Microscopy (SIM)
Structured Illumination Microscopy (SIM) enhances traditional microscopy by doubling spatial resolution through patterned light illumination and computational reconstruction, enabling visualization of cellular structures at approximately 100 nm. This super-resolution technique bridges the gap between conventional fluorescence microscopy and more complex methods like STED or PALM, offering faster imaging with lower phototoxicity suitable for live-cell observation.
Stochastic Optical Reconstruction Microscopy (STORM)
Stochastic Optical Reconstruction Microscopy (STORM) surpasses traditional microscopy by achieving nanometer-scale resolution through the precise localization of photoswitchable fluorescent probes, enabling visualization of cellular structures beyond the diffraction limit. Unlike conventional fluorescence microscopy, which is constrained by diffraction to ~200 nm resolution, STORM reconstructs high-resolution images by stochastically activating subsets of fluorophores and computationally resolving their positions with 20-30 nm precision.
Stimulated Emission Depletion (STED)
Stimulated Emission Depletion (STED) microscopy surpasses conventional microscopy by breaking the diffraction limit, achieving resolution down to 20-30 nanometers through selective deactivation of fluorophores surrounding the focal spot. This super-resolution technique enhances imaging of subcellular structures with high spatial precision, enabling detailed visualization of molecular interactions beyond traditional optical microscopy capabilities.
Photoactivated Localization Microscopy (PALM)
Microscopy traditionally provides optical imaging limited by the diffraction barrier, typically around 200 nm resolution, while Super-Resolution Microscopy techniques such as Photoactivated Localization Microscopy (PALM) surpass this limit by precisely localizing individual fluorophores to achieve resolutions below 20 nm. PALM utilizes photoactivatable fluorescent proteins to sequentially activate, image, and localize thousands of molecules, enabling detailed visualization of cellular structures at the nanoscale.
Expansion Microscopy (ExM)
Expansion Microscopy (ExM) enhances resolution beyond traditional microscopy by physically enlarging biological specimens, enabling nanoscale imaging with standard fluorescent microscopes. This technique allows detailed visualization of molecular structures and protein complexes, surpassing the diffraction limit constraints inherent in conventional and many super-resolution microscopy methods.
Single-Molecule Localization Microscopy (SMLM)
Single-Molecule Localization Microscopy (SMLM) surpasses conventional microscopy by achieving nanometer-scale resolution through precise localization of individual fluorescent molecules, enabling detailed visualization of cellular structures beyond the diffraction limit. Techniques such as PALM (Photo-Activated Localization Microscopy) and STORM (Stochastic Optical Reconstruction Microscopy) exemplify SMLM's capacity to reconstruct high-resolution images by sequentially activating and imaging sparse subsets of fluorophores.
Lattice Light Sheet Microscopy (LLSM)
Lattice Light Sheet Microscopy (LLSM) surpasses traditional fluorescence microscopy by providing high spatiotemporal resolution with reduced phototoxicity, enabling live-cell imaging at the nanoscale. This super-resolution technique uses a 2D optical lattice to illuminate specimens, achieving rapid volumetric imaging critical for dynamic biological processes.
MINFLUX Nanoscopy
MINFLUX nanoscopy achieves nanometer-scale resolution by combining fluorescence microscopy with precise localization techniques, surpassing traditional microscopy limits defined by the diffraction barrier. This super-resolution method offers localization precision down to 1-3 nanometers, enabling detailed visualization of molecular structures and dynamic processes at the nanoscale.
Adaptive Optics Microscopy
Adaptive optics microscopy enhances image resolution by correcting optical aberrations in real time, surpassing conventional microscopy limits and enabling clearer visualization of subcellular structures. Integrating adaptive optics with super-resolution techniques, such as STED or PALM, improves spatial resolution beyond the diffraction limit, crucial for precise biological and scientific analysis.
DNA-PAINT
Microscopy techniques such as DNA-PAINT leverage transient binding events of fluorescently labeled oligonucleotides to achieve spatial resolutions beyond the diffraction limit, surpassing conventional microscopy's typical resolution of approximately 200 nanometers. DNA-PAINT's ability to generate high-precision localization maps enables detailed visualization of biomolecular structures at the nanometer scale, essential for studying complex cellular processes with unparalleled clarity.
Microscopy vs Super-Resolution Microscopy Infographic
 industrydif.com
industrydif.com