Toxicology benefits from microphysiological systems (MPS) by providing more accurate human-relevant data compared to traditional animal models, enhancing drug safety assessments. Microphysiological systems mimic human tissue architecture and functions, enabling detailed analysis of toxic effects at the cellular level. These advanced platforms reduce reliance on animal testing while improving predictability and efficiency in toxicological studies.
Table of Comparison
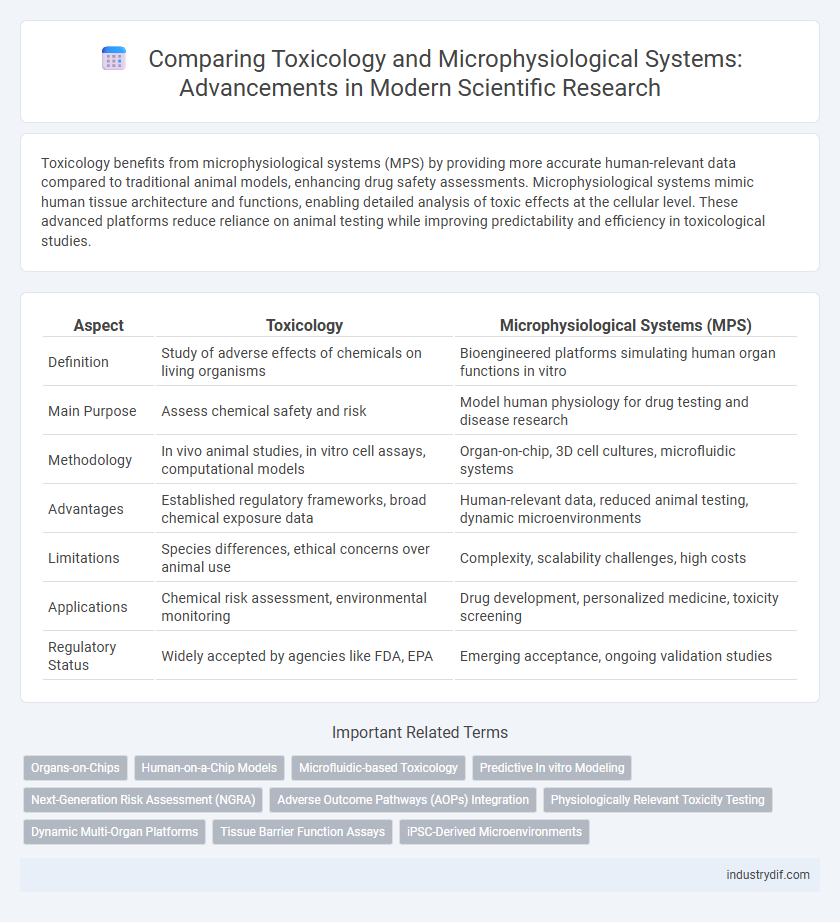
| Aspect | Toxicology | Microphysiological Systems (MPS) |
|---|---|---|
| Definition | Study of adverse effects of chemicals on living organisms | Bioengineered platforms simulating human organ functions in vitro |
| Main Purpose | Assess chemical safety and risk | Model human physiology for drug testing and disease research |
| Methodology | In vivo animal studies, in vitro cell assays, computational models | Organ-on-chip, 3D cell cultures, microfluidic systems |
| Advantages | Established regulatory frameworks, broad chemical exposure data | Human-relevant data, reduced animal testing, dynamic microenvironments |
| Limitations | Species differences, ethical concerns over animal use | Complexity, scalability challenges, high costs |
| Applications | Chemical risk assessment, environmental monitoring | Drug development, personalized medicine, toxicity screening |
| Regulatory Status | Widely accepted by agencies like FDA, EPA | Emerging acceptance, ongoing validation studies |
Introduction to Toxicology and Microphysiological Systems
Toxicology examines the adverse effects of chemical, physical, or biological agents on living organisms, emphasizing dose-response relationships and mechanisms of toxicity. Microphysiological systems (MPS) are advanced in vitro platforms that replicate human tissue and organ functions, enabling more accurate toxicity testing and disease modeling. Integration of MPS in toxicology research enhances predictive accuracy by providing human-relevant biological responses, reducing reliance on animal models.
Historical Development of Toxicology
Toxicology has evolved from ancient practices of poison evaluation to a rigorous scientific discipline integrating chemical, biological, and medical knowledge. The historical development of toxicology was marked by milestones such as Paracelsus' dose-response principle and the introduction of systematic animal testing in the 20th century. Microphysiological systems represent a recent advancement, providing human-relevant, organ-on-chip models that improve toxicological assessments by mimicking physiological responses more accurately than traditional methods.
Emergence of Microphysiological Systems in Research
Microphysiological systems (MPS) represent a cutting-edge advancement in toxicology research by providing highly accurate human tissue models that mimic in vivo conditions. These microfluidic platforms facilitate the investigation of complex biological responses to toxic substances with enhanced precision compared to traditional cell cultures or animal models. The emergence of MPS accelerates drug development and chemical safety assessments by enabling more predictive and mechanistic understanding of toxicity pathways.
Key Differences Between Toxicology and Microphysiological Systems
Toxicology primarily studies the adverse effects of chemicals and substances on living organisms through in vivo and in vitro methods, focusing on dose-response relationships and risk assessment. Microphysiological systems (MPS) are advanced, tissue-engineered models that mimic human organ functions, providing high-resolution data on cellular responses and enabling more accurate prediction of human toxicity. Unlike traditional toxicology, MPS reduce reliance on animal testing and enhance translational relevance by replicating complex human physiology at a micro-scale.
Applications of Toxicology in Industry and Academia
Toxicology plays a crucial role in both industry and academia by evaluating the safety and biological effects of chemicals, pharmaceuticals, and environmental agents on human health and ecosystems. Microphysiological systems (MPS), such as organ-on-a-chip models, are increasingly integrated into toxicology research to provide more accurate, human-relevant data on tissue-specific toxic responses and reduce reliance on animal testing. These advanced in vitro platforms enable high-throughput screening, mechanistic studies, and predictive toxicology applications critical for drug development, regulatory assessments, and mechanistic toxicological investigations.
Role of Microphysiological Systems in Drug Discovery
Microphysiological systems (MPS) simulate human organ functions, providing more accurate toxicity and efficacy data compared to traditional in vitro and animal models, thereby enhancing drug discovery processes. By replicating complex tissue interactions, MPS enable early identification of drug-induced toxicities, reducing late-stage clinical failures and improving safety profiles. Integration of MPS in toxicology accelerates lead candidate optimization and regulatory decision-making through more predictive and mechanistic insights.
Comparative Assessment: Animal Models vs Microphysiological Systems
Comparative assessment of toxicology using animal models versus microphysiological systems (MPS) reveals significant differences in predictive accuracy and ethical considerations. Animal models often present interspecies variability that limits extrapolation to human responses, whereas MPS provide human-relevant tissue architecture and microenvironment, enhancing the fidelity of toxicity evaluation. Integration of MPS into toxicological workflows promises reduction in animal usage, improved mechanistic insights, and acceleration of drug safety assessments.
Regulatory Perspectives on Toxicology and Microphysiological Systems
Regulatory agencies increasingly recognize microphysiological systems (MPS) as innovative tools enhancing toxicological assessments by providing human-relevant data that reduce reliance on animal testing. Toxicology frameworks are evolving to integrate MPS within safety evaluation protocols, emphasizing their potential to improve predictive accuracy for human responses to chemicals and pharmaceuticals. Validation and standardization efforts by entities such as the FDA and EMA aim to establish MPS as acceptable alternative methods in regulatory submissions for toxicity testing.
Technological Advancements Driving Microphysiological Systems
Microphysiological systems (MPS) represent a significant technological advancement in toxicology by providing more accurate, human-relevant models that mimic organ-level functions in vitro. Innovations in microfluidics, 3D bioprinting, and tissue engineering enable dynamic cellular environments, enhancing the predictive power of toxicity assessments compared to traditional animal models. These cutting-edge platforms facilitate mechanistic understanding of toxic responses, reducing reliance on animal testing and accelerating drug development pipelines.
Future Trends and Challenges in Toxicology and Microphysiological Systems
Emerging microphysiological systems (MPS) replicate human tissue architecture and function, offering unprecedented accuracy in toxicological assessments compared to traditional animal models. Future trends emphasize integrating multi-organ MPS platforms with advanced computational models to predict systemic toxic responses and personalized risk profiles. Challenges remain in standardizing MPS protocols, ensuring reproducibility across laboratories, and scaling production for widespread regulatory adoption in safety testing.
Related Important Terms
Organs-on-Chips
Organs-on-chips replicate human organ functions through microfluidic technology, offering precise cellular environments that enhance toxicological assessments compared to conventional in vitro and animal models. These microphysiological systems improve prediction of human responses by enabling real-time monitoring of cellular reactions to toxins across multiple interconnected tissue types.
Human-on-a-Chip Models
Human-on-a-chip models integrate multiple microphysiological systems to replicate complex human organ interactions, offering enhanced predictive accuracy for toxicology studies compared to traditional in vitro assays. These platforms enable real-time monitoring of physiological responses and improve identification of drug-induced toxicities by mimicking human metabolic and cellular environments more precisely.
Microfluidic-based Toxicology
Microfluidic-based toxicology leverages microphysiological systems (MPS) to replicate human organ functions and improve the predictive accuracy of toxicological assessments by enabling precise control of cellular microenvironments. These systems provide dynamic, high-throughput platforms that better mimic in vivo conditions compared to traditional static cell cultures, enhancing the evaluation of drug toxicity and reducing reliance on animal models.
Predictive In vitro Modeling
Microphysiological systems (MPS) enhance predictive in vitro modeling by replicating human tissue architecture and functions more accurately than traditional toxicology assays, enabling improved assessment of drug toxicity and disease mechanisms. Integration of MPS with advanced biomaterials and high-throughput screening technologies optimizes detection of cellular responses, reducing reliance on animal models and increasing translational relevance.
Next-Generation Risk Assessment (NGRA)
Microphysiological systems (MPS) provide advanced in vitro models that closely mimic human tissue responses, enhancing the accuracy of toxicology assessments within Next-Generation Risk Assessment (NGRA) frameworks. Integrating MPS with high-throughput screening and computational modeling reduces reliance on animal testing while improving predictive toxicology and safety evaluation of chemicals and pharmaceuticals.
Adverse Outcome Pathways (AOPs) Integration
The integration of Adverse Outcome Pathways (AOPs) with Microphysiological Systems (MPS) enhances toxicological assessments by providing mechanistic insights and predictive accuracy for chemical-induced adverse effects. MPS models simulate human tissue responses in vitro, enabling the mapping of molecular initiating events to downstream key events within AOP frameworks, thereby improving the relevance and translational value of toxicity testing.
Physiologically Relevant Toxicity Testing
Microphysiological systems (MPS) offer advanced physiologically relevant toxicity testing by replicating human organ functions at a microscale, enabling more accurate prediction of toxicological responses compared to traditional in vitro and animal models. These biomimetic platforms improve the assessment of drug-induced toxicity, reducing false positives and enhancing translational relevance for human safety evaluation.
Dynamic Multi-Organ Platforms
Dynamic multi-organ platforms in microphysiological systems (MPS) replicate human organ interactions with high fidelity, enabling precise toxicological assessments of drug metabolism and systemic toxicity. These platforms improve predictive accuracy over traditional methods by integrating dynamic fluid flow and organ crosstalk to model complex biological responses.
Tissue Barrier Function Assays
Microphysiological systems (MPS) provide advanced platforms that mimic human tissue barrier functions with high physiological relevance, enabling precise toxicity assessments that surpass traditional models. These systems facilitate real-time monitoring of compound permeability and barrier integrity, offering critical insights into toxicological effects on cellular junctions and tissue-specific responses.
iPSC-Derived Microenvironments
iPSC-derived microenvironments in microphysiological systems offer a highly accurate platform for toxicology testing, replicating human-specific cellular responses and reducing reliance on animal models. These systems enable precise assessment of drug toxicity and disease mechanisms by integrating pluripotent stem cell-derived tissues that mimic organ-level functions and cellular interactions.
Toxicology vs Microphysiological Systems Infographic
 industrydif.com
industrydif.com