Pharmacology examines drug interactions with biological systems to understand therapeutic effects and side effects, focusing on experimental and clinical data. Chemoinformatics applies computational techniques to analyze chemical data, enabling the prediction of molecular properties and aiding drug design. Together, these disciplines advance drug discovery by combining biological insights with data-driven chemical analysis.
Table of Comparison
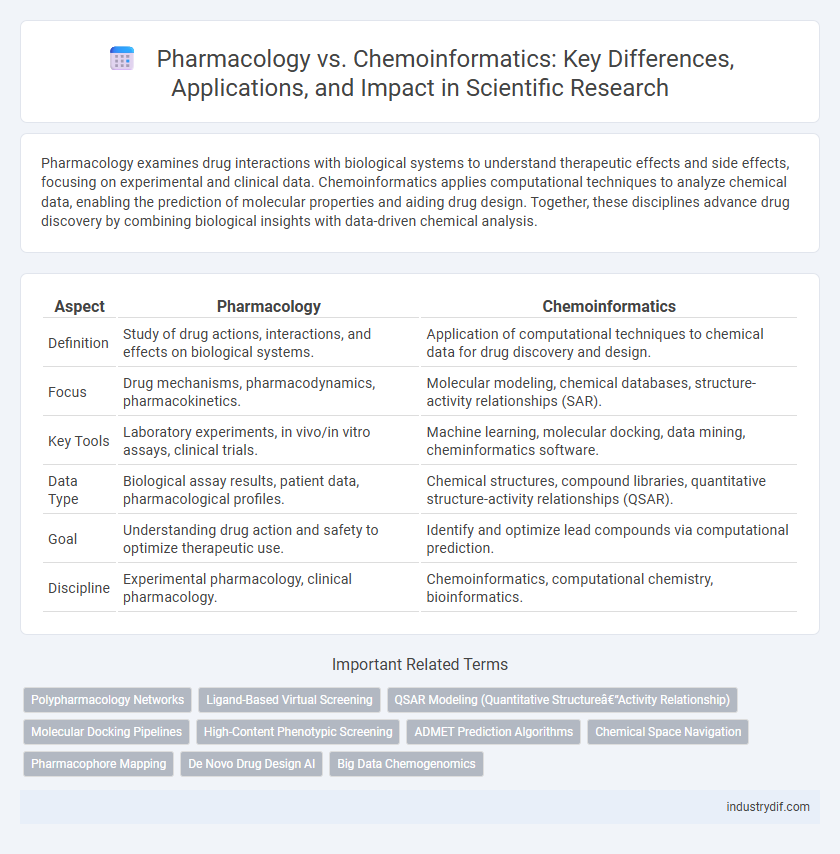
| Aspect | Pharmacology | Chemoinformatics |
|---|---|---|
| Definition | Study of drug actions, interactions, and effects on biological systems. | Application of computational techniques to chemical data for drug discovery and design. |
| Focus | Drug mechanisms, pharmacodynamics, pharmacokinetics. | Molecular modeling, chemical databases, structure-activity relationships (SAR). |
| Key Tools | Laboratory experiments, in vivo/in vitro assays, clinical trials. | Machine learning, molecular docking, data mining, cheminformatics software. |
| Data Type | Biological assay results, patient data, pharmacological profiles. | Chemical structures, compound libraries, quantitative structure-activity relationships (QSAR). |
| Goal | Understanding drug action and safety to optimize therapeutic use. | Identify and optimize lead compounds via computational prediction. |
| Discipline | Experimental pharmacology, clinical pharmacology. | Chemoinformatics, computational chemistry, bioinformatics. |
Introduction to Pharmacology and Chemoinformatics
Pharmacology studies drug action and interaction within biological systems, emphasizing mechanisms, effects, and therapeutic uses of chemicals on living organisms. Chemoinformatics applies computational techniques to analyze chemical data, supporting drug discovery and molecular design by managing large datasets and predicting biological activity. Integrating pharmacology with chemoinformatics accelerates identification of novel drug candidates through virtual screening and molecular modeling.
Key Principles of Pharmacology
Pharmacology centers on the study of drug actions, mechanisms, dose-response relationships, and pharmacokinetics, emphasizing the interaction between chemical agents and biological systems. Chemoinformatics complements this by applying computational techniques to analyze chemical data, aiding in drug discovery and molecular modeling. Key principles of pharmacology include receptor binding, drug efficacy, potency, metabolism, and the therapeutic index, which collectively guide effective and safe medication development.
Fundamentals of Chemoinformatics
Chemoinformatics integrates chemistry, computer science, and information technology to analyze chemical data for drug discovery and development. It leverages molecular modeling, data mining, and machine learning algorithms to predict compound properties and biological activities essential for pharmacological research. Distinct from traditional pharmacology, chemoinformatics emphasizes computational techniques to optimize lead compounds and enhance the efficiency of drug design processes.
Comparative Analysis: Roles in Drug Discovery
Pharmacology centers on understanding drug effects, mechanisms, and interactions within biological systems to optimize therapeutic efficacy and safety. Chemoinformatics employs computational tools and chemical data analysis to predict molecular properties, design novel compounds, and streamline drug candidate identification. Integrating pharmacological insights with chemoinformatic techniques enhances drug discovery efficiency by combining empirical biological data with predictive chemical modeling.
Data Analysis Techniques in Pharmacology vs Chemoinformatics
Pharmacology employs data analysis techniques such as dose-response modeling, pharmacokinetic/pharmacodynamic (PK/PD) modeling, and statistical hypothesis testing to evaluate drug efficacy and safety. Chemoinformatics utilizes machine learning algorithms, molecular docking simulations, and quantitative structure-activity relationship (QSAR) models to predict chemical properties and biological activity. While pharmacology emphasizes experimental data interpretation, chemoinformatics focuses on computational methods for virtual screening and drug design.
Molecular Modeling Approaches in Both Fields
Pharmacology and chemoinformatics both utilize molecular modeling approaches to understand drug interactions and optimize compound design, but their methodologies and applications differ significantly. Pharmacology employs molecular docking, quantitative structure-activity relationship (QSAR) models, and pharmacophore mapping primarily to predict drug efficacy and safety in biological systems. Chemoinformatics integrates molecular dynamics simulations, virtual screening, and descriptor-based modeling to analyze chemical properties and streamline the discovery of novel bioactive molecules.
Integrating Pharmacological Data and Chemoinformatics Tools
Integrating pharmacological data with chemoinformatics tools enhances drug discovery by enabling the analysis of complex biological interactions through computational methods. Chemoinformatics facilitates the organization, visualization, and prediction of pharmacological properties, accelerating the identification of potential drug candidates and optimizing lead compounds. This synergy improves the understanding of drug-target relationships and supports the design of more effective and safer therapeutics.
Impact on Pharmaceutical Research and Development
Pharmacology provides critical insights into drug mechanisms, efficacy, and safety through experimental and clinical studies, directly influencing dosage and therapeutic use. Chemoinformatics leverages computational tools and molecular data to optimize drug design, predict pharmacokinetics, and identify novel compounds, accelerating lead identification and reducing development costs. Integrating both fields enhances pharmaceutical research by combining empirical evidence with data-driven models, improving success rates in drug discovery and personalized medicine.
Future Trends in Pharmacology and Chemoinformatics
Emerging trends in pharmacology emphasize personalized medicine and AI-driven drug discovery, leveraging genetic and molecular data to optimize therapeutic efficacy. Chemoinformatics integrates big data analytics, machine learning algorithms, and molecular modeling to accelerate compound screening and predictive toxicology. The convergence of these fields fosters innovative approaches to precision pharmacotherapy and efficient drug development pipelines.
Conclusion: Bridging Chemistry and Biology for Drug Innovation
Pharmacology and chemoinformatics converge to accelerate drug discovery by integrating biological activity data with chemical structure analysis, enabling precise identification of therapeutic targets. Leveraging chemoinformatics tools enhances pharmacological research through predictive modeling and virtual screening, reducing the cost and time of experimental validation. This interdisciplinary approach fosters innovative drug design by uniting molecular chemistry insights with biological function, ultimately advancing personalized medicine.
Related Important Terms
Polypharmacology Networks
Pharmacology explores drug actions and interactions within biological systems, emphasizing polypharmacology networks to understand multi-target effects and therapeutic outcomes. Chemoinformatics integrates computational methods with chemical data to model and predict polypharmacology networks, enhancing drug discovery through the analysis of compound-target relationships and network pharmacology.
Ligand-Based Virtual Screening
Pharmacology investigates drug effects and mechanisms in biological systems, utilizing ligand-based virtual screening to identify potential compounds based on known bioactive ligands. Chemoinformatics applies computational techniques and molecular descriptors to analyze chemical data, enhancing ligand-based virtual screening by predicting ligand-target interactions and prioritizing drug candidates.
QSAR Modeling (Quantitative Structure–Activity Relationship)
QSAR modeling integrates pharmacological data with chemoinformatics techniques to predict biological activity based on molecular structure, enabling the design of novel compounds with optimized efficacy and reduced toxicity. This approach relies on quantitative analysis of chemical descriptors to establish mathematical relationships critical for drug discovery and development.
Molecular Docking Pipelines
Pharmacology leverages molecular docking pipelines to predict drug-receptor interactions, optimizing lead compound efficacy through computational simulations of binding affinities. Chemoinformatics enhances these pipelines by integrating large chemical databases and machine learning algorithms to improve accuracy and speed in virtual screening for potential therapeutics.
High-Content Phenotypic Screening
High-Content Phenotypic Screening integrates cellular imaging and quantitative analysis to identify bioactive compounds, bridging the gap between pharmacology's drug-target interactions and chemoinformatics' molecular data processing. This approach enables detailed phenotypic profiling, facilitating drug discovery by correlating chemical structures with complex biological responses.
ADMET Prediction Algorithms
Pharmacology relies on experimental methods to evaluate drug absorption, distribution, metabolism, excretion, and toxicity (ADMET), while chemoinformatics employs computational algorithms and machine learning models to predict ADMET properties from molecular structures. Advances in chemoinformatics-driven ADMET prediction algorithms, including deep learning and quantitative structure-activity relationship (QSAR) models, have significantly accelerated early-stage drug discovery by reducing experimental costs and improving accuracy.
Chemical Space Navigation
Pharmacology leverages chemical space navigation to identify biologically active compounds through high-throughput screening and structure-activity relationship analysis. Chemoinformatics enhances this process by utilizing computational algorithms, molecular descriptors, and virtual screening techniques to efficiently explore vast chemical spaces for drug discovery and optimization.
Pharmacophore Mapping
Pharmacophore mapping in pharmacology involves identifying the essential features responsible for a drug's biological activity, facilitating structure-based drug design. In chemoinformatics, pharmacophore models are computationally generated and analyzed to predict molecular interactions, enabling virtual screening and lead optimization.
De Novo Drug Design AI
Pharmacology focuses on understanding drug interactions with biological systems to optimize therapeutic effects, while chemoinformatics leverages computational algorithms to analyze chemical data for drug discovery. De novo drug design AI integrates both fields by using machine learning models to generate novel molecular structures with desired pharmacodynamic and pharmacokinetic properties.
Big Data Chemogenomics
Pharmacology leverages big data chemogenomics to systematically analyze drug-target interactions, enabling precision medicine through high-throughput screening and phenotypic data integration. Chemoinformatics complements this by applying computational algorithms and machine learning models to predict molecular properties and optimize chemical libraries for efficient drug discovery pipelines.
Pharmacology vs Chemoinformatics Infographic
 industrydif.com
industrydif.com