Science explores biological systems through traditional methods, focusing on hypothesis-driven experiments and mechanistic understanding. Omics technologies, such as genomics, proteomics, and metabolomics, generate large-scale data sets that provide comprehensive insights into molecular profiles and interactions. Integrating omics approaches with classical science accelerates discovery and advances personalized medicine by revealing complex biological networks.
Table of Comparison
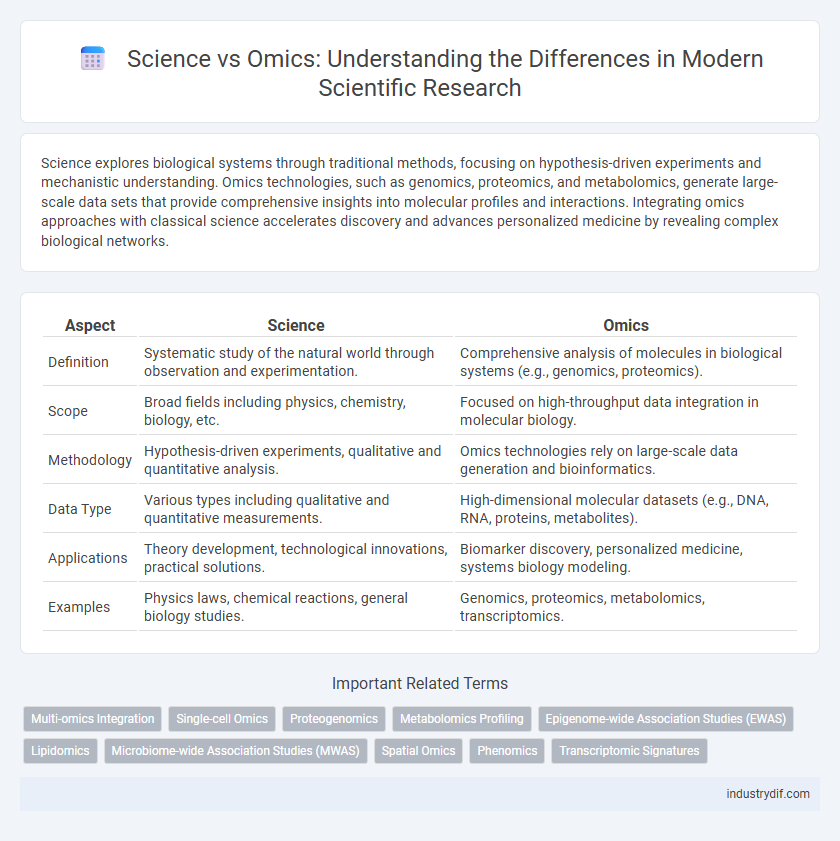
| Aspect | Science | Omics |
|---|---|---|
| Definition | Systematic study of the natural world through observation and experimentation. | Comprehensive analysis of molecules in biological systems (e.g., genomics, proteomics). |
| Scope | Broad fields including physics, chemistry, biology, etc. | Focused on high-throughput data integration in molecular biology. |
| Methodology | Hypothesis-driven experiments, qualitative and quantitative analysis. | Omics technologies rely on large-scale data generation and bioinformatics. |
| Data Type | Various types including qualitative and quantitative measurements. | High-dimensional molecular datasets (e.g., DNA, RNA, proteins, metabolites). |
| Applications | Theory development, technological innovations, practical solutions. | Biomarker discovery, personalized medicine, systems biology modeling. |
| Examples | Physics laws, chemical reactions, general biology studies. | Genomics, proteomics, metabolomics, transcriptomics. |
Defining Science and Omics: Core Concepts
Science encompasses systematic methods for acquiring knowledge through observation and experimentation, focusing on understanding natural phenomena. Omics refers to comprehensive approaches in biology that analyze collective sets of molecules, such as genomics for genes, proteomics for proteins, and metabolomics for metabolites, to elucidate complex biological systems. Defining science involves broad principles and methodologies, while omics emphasizes high-throughput data generation and integrative analysis to explore biological functions at a molecular level.
Historical Evolution: From Traditional Science to Omics
The historical evolution from traditional science to omics reflects a paradigm shift driven by advancements in high-throughput technologies and computational biology. Traditional science relied on hypothesis-driven, reductionist approaches, whereas omics encompasses large-scale, data-intensive analyses of genomes, proteomes, and metabolomes, enabling comprehensive system-level understanding. This transition has empowered researchers to decode complex biological processes and disease mechanisms with unprecedented precision.
Key Methodologies: Scientific Approaches vs Omics Technologies
Scientific approaches in research typically rely on hypothesis-driven experiments, controlled variables, and targeted analysis to elucidate specific biological mechanisms, while omics technologies encompass large-scale, high-throughput methods such as genomics, proteomics, and metabolomics to comprehensively profile biological systems. Techniques like mass spectrometry, next-generation sequencing, and bioinformatics are central to omics, enabling the simultaneous analysis of thousands of molecules to uncover complex interactions and functional networks. The integration of traditional scientific methodologies with omics technologies enhances data depth and precision, driving discoveries in systems biology and personalized medicine.
Data Generation: Hypothesis-Driven vs High-Throughput Analysis
Hypothesis-driven science relies on targeted experiments designed to test specific predictions, generating focused data sets that address precise biological questions. In contrast, omics approaches utilize high-throughput technologies, such as genomics, proteomics, and metabolomics, to produce comprehensive data that capture global molecular profiles without prior assumptions. This shift from hypothesis-driven experiments to data-intensive omics enables the discovery of novel patterns and systems-level insights by analyzing vast amounts of biological information.
Integration of Science and Omics: Bridging Traditional and Modern Approaches
Integration of science and omics technologies enhances understanding of biological systems by combining traditional experimental methods with high-throughput omics data such as genomics, proteomics, and metabolomics. This interdisciplinary approach enables comprehensive analysis of molecular mechanisms, improving disease diagnosis, personalized medicine, and biomarker discovery. Leveraging bioinformatics tools and systems biology frameworks bridges the gap between classical hypotheses and large-scale omics datasets, fostering innovation in biomedical research.
Advantages and Limitations: Science vs Omics
Science offers a broad framework for generating hypotheses, conducting experiments, and understanding biological mechanisms, while omics technologies provide high-throughput, comprehensive datasets that enable detailed molecular profiling. Omics approaches, such as genomics, proteomics, and metabolomics, excel in identifying complex biomarker patterns and uncovering system-wide interactions but often require extensive computational resources and face challenges in data interpretation and integration. The limitations of traditional scientific methods include slower data acquisition and scope constraints, whereas omics methods demand rigorous validation and sophisticated bioinformatics to translate findings into actionable biological insights.
Impact on Research and Discovery
Omics technologies, including genomics, proteomics, and metabolomics, have revolutionized scientific research by enabling comprehensive analysis of biological systems at a molecular level. This holistic approach uncovers complex interactions and molecular mechanisms that traditional science methods often overlook, accelerating discovery of biomarkers, therapeutic targets, and disease pathways. The integration of Omics data into research pipelines enhances precision medicine development and facilitates breakthroughs in personalized healthcare strategies.
Applications in Industry and Healthcare
Science and omics technologies, including genomics, proteomics, and metabolomics, drive innovation in industry and healthcare by enabling precise biomarker identification and personalized medicine. Industrial applications leverage omics data for bioengineering, improving crop yields, and optimizing microbial production processes. In healthcare, omics facilitate early disease detection, targeted therapies, and patient stratification, revolutionizing diagnostics and treatment protocols.
Challenges in Data Interpretation and Integration
Challenges in data interpretation and integration within science and omics arise from the vast heterogeneity and high dimensionality of datasets, such as genomics, proteomics, and metabolomics. Complex bioinformatics tools and machine learning algorithms are essential to decipher meaningful patterns, yet issues like data noise, missing values, and variable experimental protocols hinder accuracy. Effective integration requires standardization efforts, improved data curation, and interdisciplinary collaboration to translate multi-omics insights into actionable biological knowledge.
Future Directions: The Convergence of Science and Omics
Future directions in the convergence of science and omics emphasize integrating multi-omics data with advanced computational models to unravel complex biological systems at unprecedented resolution. Leveraging machine learning and artificial intelligence accelerates the interpretation of genomics, proteomics, metabolomics, and transcriptomics datasets, enabling personalized medicine and targeted therapies. This synthesis fosters innovative approaches to disease diagnosis, prevention, and treatment, ushering in a new era of precision science.
Related Important Terms
Multi-omics Integration
Multi-omics integration combines genomics, transcriptomics, proteomics, and metabolomics data to provide comprehensive insights into biological systems. This approach enhances the understanding of complex molecular interactions by leveraging advanced computational models and machine learning algorithms to correlate multi-layered omics datasets.
Single-cell Omics
Single-cell omics technologies enable the analysis of genomic, transcriptomic, epigenomic, and proteomic data at the resolution of individual cells, providing unprecedented insight into cellular heterogeneity and function. This contrasts with traditional bulk science approaches that average molecular signals across populations, potentially masking critical cell-specific variations essential for understanding development, disease progression, and therapeutic responses.
Proteogenomics
Proteogenomics integrates proteomic and genomic data to enhance the understanding of complex biological systems by identifying novel protein-coding regions and post-translational modifications. This approach surpasses traditional omics methods by providing a comprehensive view of gene expression and protein function, crucial for advancing personalized medicine and targeted therapies.
Metabolomics Profiling
Metabolomics profiling offers comprehensive analysis of small-molecule metabolites within biological systems, providing precise insights into cellular processes and disease mechanisms beyond traditional omics approaches such as genomics and proteomics. This technique enables identification of metabolic biomarkers, facilitating early disease detection, therapeutic monitoring, and personalized medicine advancements.
Epigenome-wide Association Studies (EWAS)
Epigenome-wide Association Studies (EWAS) systematically analyze DNA methylation patterns across the genome to identify epigenetic modifications linked to complex diseases and environmental exposures. Unlike traditional omics approaches focusing on genetic sequences or expression profiles, EWAS specifically targets epigenetic alterations, providing insights into gene regulation and disease mechanisms beyond genomic variants.
Lipidomics
Lipidomics, a branch of omics science, specifically analyzes cellular lipid profiles to understand their roles in biological systems and disease mechanisms. Unlike broader omics disciplines such as genomics or proteomics, lipidomics employs advanced mass spectrometry and bioinformatics to map lipid structures, functions, and dynamic interactions within complex biological membranes.
Microbiome-wide Association Studies (MWAS)
Microbiome-wide Association Studies (MWAS) integrate multi-omics data including metagenomics, metatranscriptomics, metabolomics, and proteomics to uncover complex interactions between microbial communities and host phenotypes. These studies leverage high-throughput sequencing and advanced bioinformatics to identify microbial biomarkers and functional pathways linked to health and disease states.
Spatial Omics
Spatial omics integrates molecular profiling with spatial context, enabling precise mapping of gene expression, proteins, and metabolites within tissue architecture. This approach surpasses traditional omics by preserving spatial information, facilitating insights into cellular heterogeneity and microenvironment interactions at unprecedented resolution.
Phenomics
Phenomics, a branch of omics, systematically quantifies phenotypes to link genetic information with observable traits, enabling comprehensive analysis of biological functions and disease mechanisms. Unlike genomics or proteomics, phenomics captures dynamic physiological changes in response to environmental factors, offering critical insights for precision medicine and crop improvement.
Transcriptomic Signatures
Transcriptomic signatures provide precise molecular profiles by analyzing RNA expression levels, offering deep insights into gene activity and regulatory mechanisms beyond traditional scientific approaches. Omics technologies, particularly transcriptomics, enable comprehensive mapping of cellular states, facilitating the identification of biomarkers and elucidation of disease pathways with high resolution.
Science vs Omics Infographic
 industrydif.com
industrydif.com