Clinical trials remain the gold standard for evaluating new treatments in scientific pet research, providing direct evidence of efficacy and safety through real-world testing on animal subjects. Digital twins complement this by creating virtual models of pets that simulate biological responses, enabling researchers to predict outcomes and optimize treatment plans without ethical concerns or delays. Combining clinical trials with digital twin technology accelerates innovation, reduces costs, and enhances personalized veterinary care.
Table of Comparison
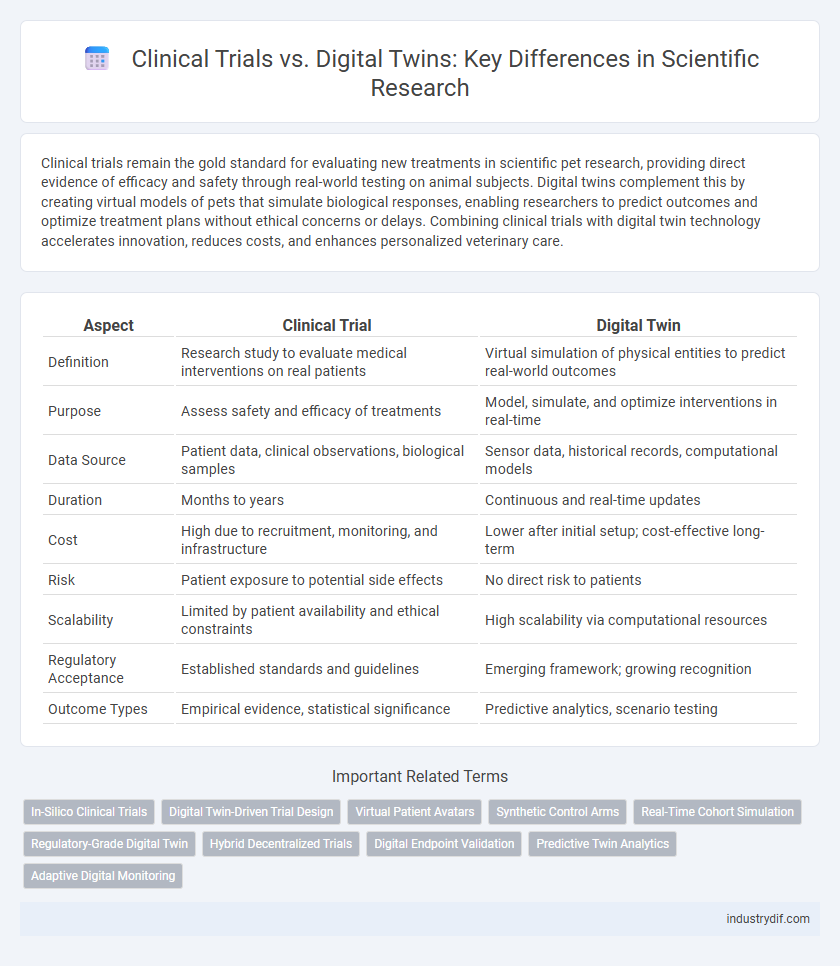
| Aspect | Clinical Trial | Digital Twin |
|---|---|---|
| Definition | Research study to evaluate medical interventions on real patients | Virtual simulation of physical entities to predict real-world outcomes |
| Purpose | Assess safety and efficacy of treatments | Model, simulate, and optimize interventions in real-time |
| Data Source | Patient data, clinical observations, biological samples | Sensor data, historical records, computational models |
| Duration | Months to years | Continuous and real-time updates |
| Cost | High due to recruitment, monitoring, and infrastructure | Lower after initial setup; cost-effective long-term |
| Risk | Patient exposure to potential side effects | No direct risk to patients |
| Scalability | Limited by patient availability and ethical constraints | High scalability via computational resources |
| Regulatory Acceptance | Established standards and guidelines | Emerging framework; growing recognition |
| Outcome Types | Empirical evidence, statistical significance | Predictive analytics, scenario testing |
Introduction to Clinical Trials and Digital Twins
Clinical trials are research studies conducted with human participants to evaluate the safety and efficacy of new medical treatments or interventions under controlled conditions. Digital twins, virtual replicas of physical entities or systems, simulate biological processes and patient responses using real-time data and advanced computational models. Integrating digital twins into clinical trials enhances predictive accuracy, optimizes trial designs, and accelerates drug development by providing personalized insights and reducing reliance on traditional trial methods.
Key Definitions: Clinical Trials vs Digital Twins
Clinical trials are structured research studies involving human participants to evaluate the safety and efficacy of medical interventions under controlled conditions. Digital twins refer to virtual replicas of physical systems, including biological organisms, created using real-time data and advanced simulations to predict outcomes and optimize treatments. While clinical trials rely on empirical testing with participants, digital twins enable personalized, data-driven experimentation and analysis in silico.
Historical Evolution of Clinical Trials
Clinical trials have evolved from rudimentary observational studies in the 18th century to highly regulated, randomized controlled trials (RCTs) that ensure robust data integrity and patient safety. The integration of digital twin technology offers a transformative approach by creating virtual patient models that simulate biological responses, enhancing trial precision and reducing time and costs. This shift reflects a broader trend toward leveraging advanced computational methods in clinical research to optimize therapeutic development and personalized medicine.
Emergence of Digital Twin Technology in Healthcare
Digital twin technology in healthcare simulates patient-specific models using real-time data to enhance precision in diagnosis and treatment, surpassing traditional clinical trials in agility and personalization. Unlike clinical trials with fixed protocols and limited sample sizes, digital twins enable continuous monitoring and adaptive interventions, accelerating drug development and improving patient outcomes. The integration of digital twins leverages AI and IoT devices, transforming healthcare decision-making by providing dynamic, data-driven insights that reduce costs and trial durations.
Comparative Methodologies: Traditional vs Virtual Modeling
Clinical trials rely on physical participant recruitment and controlled environments to assess drug efficacy and safety, whereas digital twins utilize virtual modeling to simulate patient responses and predict outcomes through advanced computational algorithms. The traditional methodology provides empirical data grounded in real-world biological variability, while virtual modeling enables rapid hypothesis testing and personalized treatment scenarios without exposing patients to potential risks. Integrating digital twins with clinical trials enhances precision medicine by combining robust empirical evidence with dynamic, patient-specific simulations.
Data Integration and Management in Both Approaches
Clinical trials rely on structured data collection from human subjects, emphasizing standardized protocols for patient data integration and regulatory-compliant data management systems to ensure accuracy and traceability. Digital twins synthesize multi-source data, including real-time biomedical sensor inputs and historical clinical datasets, enabling dynamic simulation and predictive modeling through advanced data fusion techniques. Both approaches require robust data governance frameworks, but digital twins offer enhanced capabilities for integrating heterogeneous data streams to support personalized medicine and adaptive clinical decision-making.
Regulatory Challenges: Clinical Trial Protocols vs Digital Twin Validation
Clinical trial protocols are governed by stringent regulatory frameworks ensuring patient safety and data integrity through predefined study designs and endpoint criteria. In contrast, digital twin validation faces regulatory challenges related to model accuracy, reproducibility, and real-world data integration, requiring novel standards for computational simulations. Regulatory agencies are actively developing guidelines to harmonize digital twin validation with traditional clinical trial requirements, emphasizing transparency and reliability in patient-specific predictions.
Patient Safety and Ethical Considerations
Clinical trials remain the gold standard for assessing new medical treatments but pose challenges in patient safety due to potential adverse effects and ethical dilemmas involving informed consent and risk exposure. Digital twins, virtual replicas of patients, enable precise simulation of treatment outcomes in a controlled environment, significantly reducing risks and ethical concerns by minimizing real-world harm. Integrating digital twin technology with clinical trials enhances patient safety by providing personalized predictions while upholding rigorous ethical standards in medical research.
Impact on Drug Development Timelines and Costs
Clinical trials remain the gold standard for evaluating drug safety and efficacy but often require extensive timeframes and substantial financial investment, frequently spanning several years and costing hundreds of millions of dollars. Digital twin technology, by creating precise virtual replicas of human physiology and patient-specific responses, accelerates drug development by enabling in silico testing, which significantly reduces the number and duration of clinical trials needed. Integrating digital twins into drug development workflows can decrease costs by up to 30% and shorten development timelines by 20-50%, enhancing efficiency without compromising data reliability.
Future Perspectives: Hybrid Models and Industry Trends
Hybrid models combining clinical trials with digital twin technology are poised to revolutionize personalized medicine by enabling real-time simulation of patient responses and optimizing trial protocols. Industry trends indicate increased investment in AI-driven digital twins to enhance predictive accuracy, reduce trial costs, and accelerate drug development timelines. Future perspectives highlight the integration of multi-omics data and advanced computational algorithms to create more robust, adaptive models that improve patient stratification and treatment efficacy.
Related Important Terms
In-Silico Clinical Trials
In-silico clinical trials leverage digital twins to simulate patient responses, enhancing drug development efficiency by reducing reliance on traditional human trials and enabling personalized medicine insights. These computational models offer scalable, cost-effective platforms for testing therapeutic outcomes and predicting adverse effects, ultimately accelerating regulatory approvals.
Digital Twin-Driven Trial Design
Digital Twin-driven trial design leverages real-time patient data and advanced simulations to create highly personalized and adaptive clinical trial models, enhancing accuracy and reducing trial duration. This approach enables dynamic adjustments based on virtual patient responses, optimizing resource allocation and improving predictive validity compared to traditional clinical trials.
Virtual Patient Avatars
Virtual patient avatars in clinical trials provide a dynamic, data-driven model to simulate individual responses to treatments, enhancing precision medicine and reducing trial costs. Unlike traditional clinical trials, digital twins enable continuous real-time monitoring and personalized adjustments by integrating multi-omics data and physiological parameters.
Synthetic Control Arms
Clinical trials traditionally rely on control arms to compare treatment efficacy, whereas digital twins enhance this process by creating Synthetic Control Arms through computational models simulating patient responses. These Synthetic Control Arms leverage real-world data and machine learning algorithms to reduce the need for placebo groups, accelerate trial timelines, and improve personalized medicine strategies.
Real-Time Cohort Simulation
Real-time cohort simulation using digital twins enables dynamic modeling of patient populations by integrating real-time data streams, enhancing predictive accuracy beyond traditional clinical trial protocols. This approach accelerates decision-making and optimizes trial design through continuous virtual patient monitoring and scenario testing.
Regulatory-Grade Digital Twin
A Regulatory-Grade Digital Twin offers real-time, patient-specific simulations that complement traditional clinical trial data by enabling dynamic modeling of drug responses and disease progression under regulatory-compliant standards. This approach enhances precision in clinical decision-making and accelerates regulatory approval by validating trial hypotheses through continuous, adaptive virtual testing environments.
Hybrid Decentralized Trials
Hybrid decentralized trials leverage the integration of clinical trials with digital twin technology to enhance real-time patient monitoring and personalized treatment simulations. This approach improves data accuracy, reduces trial costs, and accelerates drug development by combining remote patient engagement with virtual modeling.
Digital Endpoint Validation
Digital endpoint validation in digital twins enables continuous monitoring and real-time data acquisition, offering precision and adaptability beyond traditional clinical trials. This approach enhances predictive accuracy for patient outcomes by simulating physiological responses under varied conditions, reducing reliance on static trial endpoints.
Predictive Twin Analytics
Predictive twin analytics leverages digital twin technology to simulate patient-specific clinical scenarios, enhancing the precision of outcome predictions beyond traditional clinical trial methods. This approach enables real-time data integration and continuous model refinement, significantly improving personalized treatment planning and risk assessment in biomedical research.
Adaptive Digital Monitoring
Adaptive digital monitoring leverages real-time data from digital twins to enhance clinical trial precision by simulating patient responses and optimizing treatment protocols dynamically. This approach reduces trial duration and improves outcome predictability, surpassing traditional clinical trial methodologies in efficiency and personalized intervention.
Clinical Trial vs Digital Twin Infographic
 industrydif.com
industrydif.com